Monday Poster Session
Category: Biliary/Pancreas
P1757 - Time to Initiation of the Second-Line Therapy Obeticholic Acid in Patients With Primary Biliary Cholangitis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Robert S. Brown, Jr.
Weill Cornell Medical College
New York, NY
Presenting Author(s)
Sonal Kumar, MD, MPH1, Joanna P. MacEwan, PhD2, Alina Levine, 3, Leona Bessonova, PhD4, Radhika Nair, PhD4, Darren Wheeler, PhD4, Jing Li, PhD4, Robert S. Brown, 1
1Weill Cornell Medical College, New York, NY; 2Genesis Research Group, Hoboken, NJ; 3Genesis Research, Hoboken, NJ; 4Intercept Pharmaceuticals, Inc., Morristown, NJ
Introduction: Obeticholic acid (OCA) is the only approved second-line (2L) therapy for patients with primary biliary cholangitis (PBC). We estimated the time from 2L therapy eligibility to the initiation of OCA in patients with PBC.
Methods: Patients with PBC (1 inpatient or ≥ 2 outpatient claims) eligible for 2L therapy (defined as inadequate response to ursodeoxycholic acid) between June 2016 and February 2022 were identified from Komodo Health. Index was the first date of 2L eligibility. Patients were enrolled for ≥ 12 months pre-index and were ≥ 18 years old at index. They were followed until OCA initiation, end of insurance enrollment, long-term care admission, death, or end of study (May 2023), whichever occurred first.
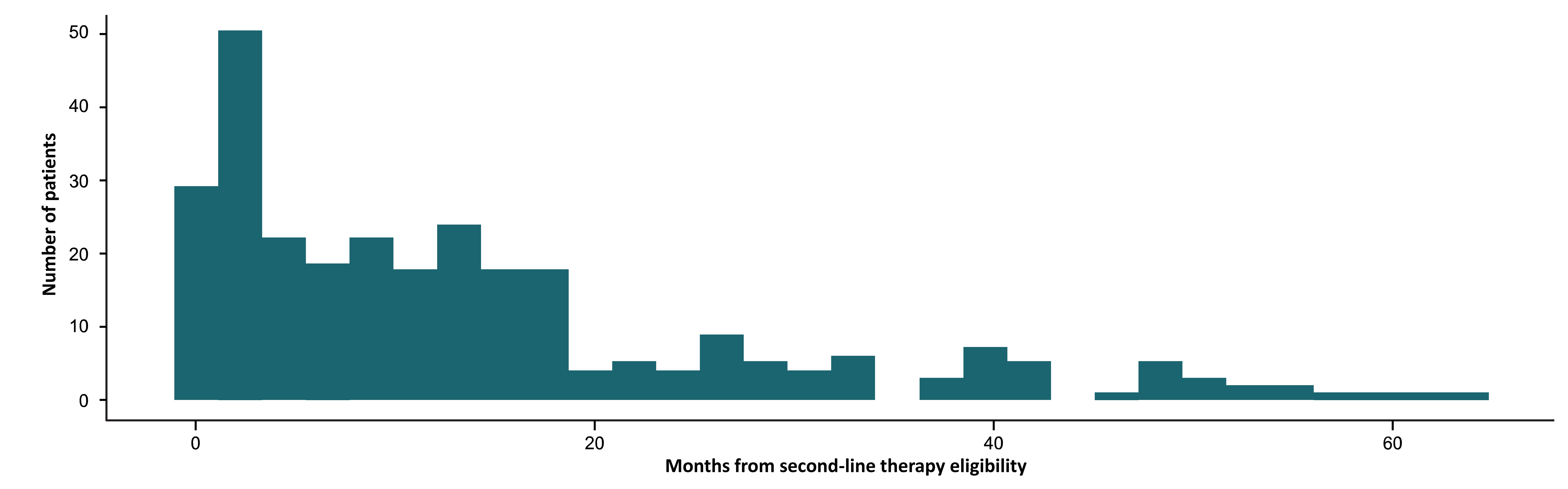
Results: Of 3880 patients eligible for 2L therapy, 306 (7.9%) initiated OCA, and 3574 (92.1%) did not. Eligible patients had a mean (SD) age of 61.0 years (11.4), were mostly female (90.8%), and about half were White (50.5%). The mean (SD) time to OCA initiation from 2L eligibility was 14.2 (14.3) months, with a median (interquartile range) of 10.2 (2.8–18.1) months (Fig. 1). Patients initiating OCA were slightly younger than those who did not, with mean (SD) ages of 57.2 (10.2) vs 61.3 (11.4) years, respectively. Among those initiating OCA, 90.7% were female, and 42.1% were White. During the 1-year baseline period (12 months before and including index date), a higher proportion of patients initiating OCA had cirrhosis (17.6% vs 12.4% who did not initiate OCA) and other autoimmune diseases in addition to PBC (29.3% vs 26.3%). At the time of OCA initiation, the proportion of patients with cirrhosis almost doubled compared with when patients were first eligible for 2L therapy (17.6% to 31.4%). Patients initiating OCA had higher mean (SD) baseline alkaline phosphatase (ALP) levels (251.9 [132.7] IU/L) than those who did not (184.7 [99.2] IU/L). At the time of 2L eligibility, 55.5% of patients initiating OCA had baseline ALP levels ≥ 1.67 × upper limit of normal (ULN) vs 25.3% of those who did not initiate OCA; by the time of OCA initiation, 65.3% of the patients had ALP > 1.67 × ULN.
Discussion: Among eligible patients, less than 10% initiated OCA as 2L therapy, with a considerable delay observed. This delay suggests patients may experience disease progression, including cirrhosis, before starting 2L therapy. Timely initiation of OCA in appropriate patients could enhance its clinical benefit on long-term outcomes.
©2023 Komodo Health, Inc. All rights reserved. Used with permission.
Disclosures:
Sonal Kumar, MD, MPH1, Joanna P. MacEwan, PhD2, Alina Levine, 3, Leona Bessonova, PhD4, Radhika Nair, PhD4, Darren Wheeler, PhD4, Jing Li, PhD4, Robert S. Brown, 1. P1757 - Time to Initiation of the Second-Line Therapy Obeticholic Acid in Patients With Primary Biliary Cholangitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Weill Cornell Medical College, New York, NY; 2Genesis Research Group, Hoboken, NJ; 3Genesis Research, Hoboken, NJ; 4Intercept Pharmaceuticals, Inc., Morristown, NJ
Introduction: Obeticholic acid (OCA) is the only approved second-line (2L) therapy for patients with primary biliary cholangitis (PBC). We estimated the time from 2L therapy eligibility to the initiation of OCA in patients with PBC.
Methods: Patients with PBC (1 inpatient or ≥ 2 outpatient claims) eligible for 2L therapy (defined as inadequate response to ursodeoxycholic acid) between June 2016 and February 2022 were identified from Komodo Health. Index was the first date of 2L eligibility. Patients were enrolled for ≥ 12 months pre-index and were ≥ 18 years old at index. They were followed until OCA initiation, end of insurance enrollment, long-term care admission, death, or end of study (May 2023), whichever occurred first.
Results: Of 3880 patients eligible for 2L therapy, 306 (7.9%) initiated OCA, and 3574 (92.1%) did not. Eligible patients had a mean (SD) age of 61.0 years (11.4), were mostly female (90.8%), and about half were White (50.5%). The mean (SD) time to OCA initiation from 2L eligibility was 14.2 (14.3) months, with a median (interquartile range) of 10.2 (2.8–18.1) months (Fig. 1). Patients initiating OCA were slightly younger than those who did not, with mean (SD) ages of 57.2 (10.2) vs 61.3 (11.4) years, respectively. Among those initiating OCA, 90.7% were female, and 42.1% were White. During the 1-year baseline period (12 months before and including index date), a higher proportion of patients initiating OCA had cirrhosis (17.6% vs 12.4% who did not initiate OCA) and other autoimmune diseases in addition to PBC (29.3% vs 26.3%). At the time of OCA initiation, the proportion of patients with cirrhosis almost doubled compared with when patients were first eligible for 2L therapy (17.6% to 31.4%). Patients initiating OCA had higher mean (SD) baseline alkaline phosphatase (ALP) levels (251.9 [132.7] IU/L) than those who did not (184.7 [99.2] IU/L). At the time of 2L eligibility, 55.5% of patients initiating OCA had baseline ALP levels ≥ 1.67 × upper limit of normal (ULN) vs 25.3% of those who did not initiate OCA; by the time of OCA initiation, 65.3% of the patients had ALP > 1.67 × ULN.
Discussion: Among eligible patients, less than 10% initiated OCA as 2L therapy, with a considerable delay observed. This delay suggests patients may experience disease progression, including cirrhosis, before starting 2L therapy. Timely initiation of OCA in appropriate patients could enhance its clinical benefit on long-term outcomes.
©2023 Komodo Health, Inc. All rights reserved. Used with permission.

Figure: Figure 1. Time to OCA initiation from second-line therapy eligibility.
Abbreviation: OCA, obeticholic acid.
Abbreviation: OCA, obeticholic acid.
Disclosures:
Sonal Kumar: Intercept Pharmaceuticals, Inc. – Advisory Committee/Board Member, Speaker and site PI.
Joanna MacEwan: Genesis Research Group – Employee.
Alina Levine: Genesis Research Group – Employee.
Leona Bessonova: Intercept Pharmaceuticals, Inc. – Employee.
Radhika Nair: Intercept Pharmaceuticals, Inc. – Employee.
Darren Wheeler: Intercept Pharmaceuticals, Inc. – Employee.
Jing Li: Intercept Pharmaceuticals Inc. – Employee.
Robert Brown: AbbVie – Consultant. Gilead – Consultant. Intercept – Consultant. Mallinckrodt – Consultant.
Sonal Kumar, MD, MPH1, Joanna P. MacEwan, PhD2, Alina Levine, 3, Leona Bessonova, PhD4, Radhika Nair, PhD4, Darren Wheeler, PhD4, Jing Li, PhD4, Robert S. Brown, 1. P1757 - Time to Initiation of the Second-Line Therapy Obeticholic Acid in Patients With Primary Biliary Cholangitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
