Monday Poster Session
Category: Biliary/Pancreas
P1760 - A Paired Comparison of Multiple Pancreatic Cyst Fluid Analyses
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- DM
Daniel Marino, MD, MBA
NYU Langone Health
New York, NY
Presenting Author(s)
Daniel Marino, MD, MBA1, Rong Xia, MD, PhD1, Prahan Chetlur, MD1, Lauren Khanna, MD2, Eileen Janec, MD2, Gregory B. Haber, MD2, Claudine Kipp, PA1, Tamas A.. Gonda, MD2
1NYU Langone Health, New York, NY; 2NYU Grossman School of Medicine, New York, NY
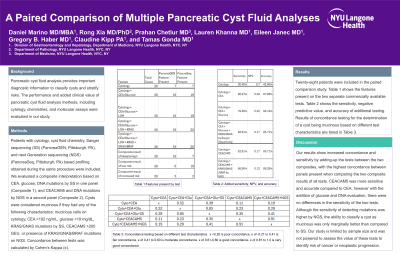
Introduction: Pancreatic cyst fluid analysis provides important diagnostic information to classify cysts and stratify risks. The performance and added clinical value of pancreatic cyst fluid analysis methods, including cytology, chemistries, and molecular assays were evaluated in our study.
Methods: Patients with cytology, cyst fluid chemistry, Sanger sequencing (SS) (PancreaGEN, Pittsburgh, PA), and next Generation sequencing (NGS) (PancreaSeq, Pittsburgh, PA) based profiling obtained during the same procedure were included. We evaluated a composite interpretation based on CEA, glucose (glu), DNA mutations by SS in one panel (Composite 1), and CEACAM5 and DNA mutations by NGS in a second panel (Composite 2). Cysts were considered mucinous if they had any of the following characteristics: mucinous cells on cytology (cyto), CEA >192 ng/mL, glu < 10 mg/dL, KRAS/GNAS mutations by SS, CEACAM5 >200 GEU, or presence of KRAS/GNAS/BRAF mutations on NGS. Concordance between test characteristics was calculated by Cohen’s Kappa (κ).
Results: Twenty-eight patients were included in the paired comparison study. Results of concordance testing for the determination of a cyst being mucinous based on different test characteristics are listed in Table 1. The concordance between tests for mucinous criteria based on cyto+CEA+glu and cyto+CEACAM5 was fair (κ=0.23). The inclusion of SS into the cyto+CEA+glu testing and NGS into the cyto+CEACAM5 testing increased the concordance (κ=0.41) The concordance for test generated risk assessments between Composite 1 and 2 was fair (κ=0.25). Sensitivity for detecting mucinous lesions was 61% by cyto+CEA, 78% by cyto+CEA+glu, 83% by cyto+CEA+glu+SS (Composite 1), 83% by cyto+CEACAM5, and 87% by cyto+CEACAM5+NGS (Composite 2).
Discussion: Our results show increased concordance and sensitivity by adding-up the tests between the two composites, with the highest concordance between panels present when comparing the two composite results of all tests. CEACAM5 was more sensitive and accurate compared to CEA, however with the addition of glu and DNA evaluation, there were no differences in the sensitivity of the two tests. Although the sensitivity of detecting mutations was higher by NGS, the ability to classify a cyst as mucinous was only marginally better than compared to SS. Our study is limited by sample size and was not powered to assess the value of these tests to identify risk of cancer or neoplastic progression.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Daniel Marino, MD, MBA1, Rong Xia, MD, PhD1, Prahan Chetlur, MD1, Lauren Khanna, MD2, Eileen Janec, MD2, Gregory B. Haber, MD2, Claudine Kipp, PA1, Tamas A.. Gonda, MD2. P1760 - A Paired Comparison of Multiple Pancreatic Cyst Fluid Analyses, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1NYU Langone Health, New York, NY; 2NYU Grossman School of Medicine, New York, NY
Introduction: Pancreatic cyst fluid analysis provides important diagnostic information to classify cysts and stratify risks. The performance and added clinical value of pancreatic cyst fluid analysis methods, including cytology, chemistries, and molecular assays were evaluated in our study.
Methods: Patients with cytology, cyst fluid chemistry, Sanger sequencing (SS) (PancreaGEN, Pittsburgh, PA), and next Generation sequencing (NGS) (PancreaSeq, Pittsburgh, PA) based profiling obtained during the same procedure were included. We evaluated a composite interpretation based on CEA, glucose (glu), DNA mutations by SS in one panel (Composite 1), and CEACAM5 and DNA mutations by NGS in a second panel (Composite 2). Cysts were considered mucinous if they had any of the following characteristics: mucinous cells on cytology (cyto), CEA >192 ng/mL, glu < 10 mg/dL, KRAS/GNAS mutations by SS, CEACAM5 >200 GEU, or presence of KRAS/GNAS/BRAF mutations on NGS. Concordance between test characteristics was calculated by Cohen’s Kappa (κ).
Results: Twenty-eight patients were included in the paired comparison study. Results of concordance testing for the determination of a cyst being mucinous based on different test characteristics are listed in Table 1. The concordance between tests for mucinous criteria based on cyto+CEA+glu and cyto+CEACAM5 was fair (κ=0.23). The inclusion of SS into the cyto+CEA+glu testing and NGS into the cyto+CEACAM5 testing increased the concordance (κ=0.41) The concordance for test generated risk assessments between Composite 1 and 2 was fair (κ=0.25). Sensitivity for detecting mucinous lesions was 61% by cyto+CEA, 78% by cyto+CEA+glu, 83% by cyto+CEA+glu+SS (Composite 1), 83% by cyto+CEACAM5, and 87% by cyto+CEACAM5+NGS (Composite 2).
Discussion: Our results show increased concordance and sensitivity by adding-up the tests between the two composites, with the highest concordance between panels present when comparing the two composite results of all tests. CEACAM5 was more sensitive and accurate compared to CEA, however with the addition of glu and DNA evaluation, there were no differences in the sensitivity of the two tests. Although the sensitivity of detecting mutations was higher by NGS, the ability to classify a cyst as mucinous was only marginally better than compared to SS. Our study is limited by sample size and was not powered to assess the value of these tests to identify risk of cancer or neoplastic progression.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Daniel Marino indicated no relevant financial relationships.
Rong Xia indicated no relevant financial relationships.
Prahan Chetlur indicated no relevant financial relationships.
Lauren Khanna indicated no relevant financial relationships.
Eileen Janec indicated no relevant financial relationships.
Gregory Haber indicated no relevant financial relationships.
Claudine Kipp indicated no relevant financial relationships.
Tamas Gonda indicated no relevant financial relationships.
Daniel Marino, MD, MBA1, Rong Xia, MD, PhD1, Prahan Chetlur, MD1, Lauren Khanna, MD2, Eileen Janec, MD2, Gregory B. Haber, MD2, Claudine Kipp, PA1, Tamas A.. Gonda, MD2. P1760 - A Paired Comparison of Multiple Pancreatic Cyst Fluid Analyses, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
