Monday Poster Session
Category: Colon
P1933 - Completeness of Reporting of Patient-Reported Outcomes in Clinical Trials for Colostomy and Ileostomy Interventions
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Ian Fladie, DO
Baylor Scott & White Medical Center
Temple, TX
Presenting Author(s)
Ian Fladie, DO1, Audrey Wise, DO1, John Whelan, DO2, Cameron Lenz, DO3, Ross Nowlin, DO4, James Fleshman, MD5, Micah Hartwell, PhD6, Matt Vassar, PhD6
1Baylor Scott & White Medical Center, Temple, TX; 2University of Oklahoma College of Medicine, Oklahoma City, OK; 3CHRISTUS Highland Medical Center, Tyler, TX; 4Medical College of Georgia at Augusta University, Augusta, GA; 5Baylor Scott & White Medical Center, Dallas, TX; 6Oklahoma State University College of Medicine, Tulsa, OK
Introduction: Annually nearly 120,000 patients undergo surgical procedures resulting in ostomy formation. In recent years, several studies have highlighted the association between ostomies and how it negatively effects quality of life. Given that quality of life is a main concern for individuals with ostomies, the need to incorporate patient perspective within clinical trials (CTs) is essential. Our primary aim was to assess patient reported outcome (PRO) adherence among colostomy- and ileostomy-related clinical trials to the International Society for Quality-of-Life Research (ISOQOL) reporting guidelines.
Methods: This cross-sectional analysis used MEDLINE and Embase to identify colostomy- and ileostomy-related clinical trials that included patient reported outcomes. Data were extracted for study characteristics and ISOQOL checklist adherence. Concordance was assessed for all studies and was achieved if the PRO measure included items that were (1) meaningful and relevant to the hypothesized variable (face validity), from which (2) a score could be generated and (3) was appropriately reported.
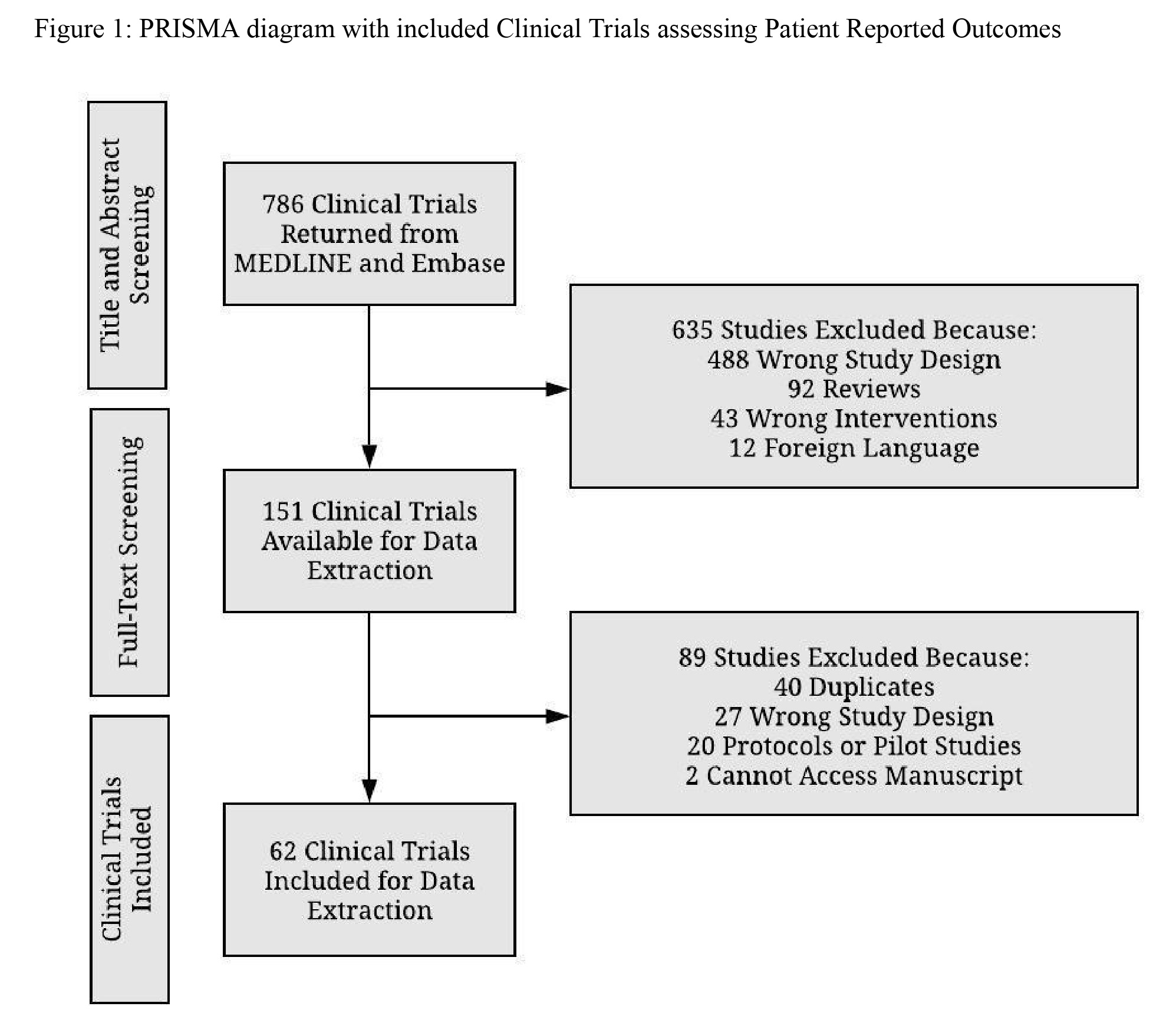
Results: Our study returned 786 articles and our final sample included 62 clinical trials after screening. Among the 62 clinical trials, 31 (31/62; 50%) achieved two-thirds completeness of ISOQOL guidelines with over three-fourths of the trials (58/62; 93.5%) included PRO assessment tools (item number 10) within their manuscript. Furthermore, 43 (43/62; 69.4%) clinical trials met concordance. Among the included studies, those which stated the PRO as a primary or secondary endpoint were 11.1% more complete in their reporting (t = 2.11, p = 0.039).
Discussion: Our findings demonstrate Clinical Trials on colostomy and ileostomy interventions have greater ISOQOL reporting adherence when a PRO measure is specified as a primary or secondary outcome. Such specifications may increase the quality of reporting on Clinical Trials evaluating colostomies and ileostomies. Ultimately, Clinical Trials with scientific rigor aimed at addressing PRO would benefit the research community and patients with ostomies at large. Given the increase of PRO inclusion in clinical trials, we advocate for the standardization of PRO reporting as findings from Clinical Trials may ultimately influence subsequent trials or clinical practice guidelines and the use of validated reporting guidelines such as ISOQOL.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Ian Fladie, DO1, Audrey Wise, DO1, John Whelan, DO2, Cameron Lenz, DO3, Ross Nowlin, DO4, James Fleshman, MD5, Micah Hartwell, PhD6, Matt Vassar, PhD6. P1933 - Completeness of Reporting of Patient-Reported Outcomes in Clinical Trials for Colostomy and Ileostomy Interventions, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Baylor Scott & White Medical Center, Temple, TX; 2University of Oklahoma College of Medicine, Oklahoma City, OK; 3CHRISTUS Highland Medical Center, Tyler, TX; 4Medical College of Georgia at Augusta University, Augusta, GA; 5Baylor Scott & White Medical Center, Dallas, TX; 6Oklahoma State University College of Medicine, Tulsa, OK
Introduction: Annually nearly 120,000 patients undergo surgical procedures resulting in ostomy formation. In recent years, several studies have highlighted the association between ostomies and how it negatively effects quality of life. Given that quality of life is a main concern for individuals with ostomies, the need to incorporate patient perspective within clinical trials (CTs) is essential. Our primary aim was to assess patient reported outcome (PRO) adherence among colostomy- and ileostomy-related clinical trials to the International Society for Quality-of-Life Research (ISOQOL) reporting guidelines.
Methods: This cross-sectional analysis used MEDLINE and Embase to identify colostomy- and ileostomy-related clinical trials that included patient reported outcomes. Data were extracted for study characteristics and ISOQOL checklist adherence. Concordance was assessed for all studies and was achieved if the PRO measure included items that were (1) meaningful and relevant to the hypothesized variable (face validity), from which (2) a score could be generated and (3) was appropriately reported.
Results: Our study returned 786 articles and our final sample included 62 clinical trials after screening. Among the 62 clinical trials, 31 (31/62; 50%) achieved two-thirds completeness of ISOQOL guidelines with over three-fourths of the trials (58/62; 93.5%) included PRO assessment tools (item number 10) within their manuscript. Furthermore, 43 (43/62; 69.4%) clinical trials met concordance. Among the included studies, those which stated the PRO as a primary or secondary endpoint were 11.1% more complete in their reporting (t = 2.11, p = 0.039).
Discussion: Our findings demonstrate Clinical Trials on colostomy and ileostomy interventions have greater ISOQOL reporting adherence when a PRO measure is specified as a primary or secondary outcome. Such specifications may increase the quality of reporting on Clinical Trials evaluating colostomies and ileostomies. Ultimately, Clinical Trials with scientific rigor aimed at addressing PRO would benefit the research community and patients with ostomies at large. Given the increase of PRO inclusion in clinical trials, we advocate for the standardization of PRO reporting as findings from Clinical Trials may ultimately influence subsequent trials or clinical practice guidelines and the use of validated reporting guidelines such as ISOQOL.

Figure: PRISMA diagram with included Clinical Trials assessing Patient Reported Outcomes
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Ian Fladie indicated no relevant financial relationships.
Audrey Wise indicated no relevant financial relationships.
John Whelan indicated no relevant financial relationships.
Cameron Lenz indicated no relevant financial relationships.
Ross Nowlin indicated no relevant financial relationships.
James Fleshman indicated no relevant financial relationships.
Micah Hartwell indicated no relevant financial relationships.
Matt Vassar indicated no relevant financial relationships.
Ian Fladie, DO1, Audrey Wise, DO1, John Whelan, DO2, Cameron Lenz, DO3, Ross Nowlin, DO4, James Fleshman, MD5, Micah Hartwell, PhD6, Matt Vassar, PhD6. P1933 - Completeness of Reporting of Patient-Reported Outcomes in Clinical Trials for Colostomy and Ileostomy Interventions, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
