Monday Poster Session
Category: Colon
P2056 - Treatment of Immune Checkpoint Inhibitor Induced Sclerosing Mesenteritis: A Case Series
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Abdullah S. Aleem, MD
University of Texas at Houston
Houston, TX
Presenting Author(s)
Abdullah S. Aleem, MD1, Yinghong Wang, MD, PhD2
1University of Texas at Houston, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX
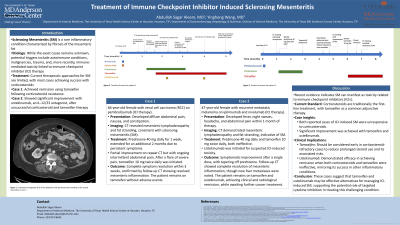
Introduction: Sclerosing mesenteritis (SM) is a rare condition involving nonspecific inflammation and fibrosis of mesenteric fat. While its etiology is unclear, it is associated with autoimmune processes, malignancy, trauma, and and more recently immune mediated toxicity following immune checkpoint inhibitor (ICI) cancer therapy. We report two cases: one achieving remission with tamoxifen after corticosteroid failure, and another improving with ustekinumab, an interleukin-12 and -23 antagonist, after failing both corticosteroids and tamoxifen.
Case Description/Methods: Patient 1:
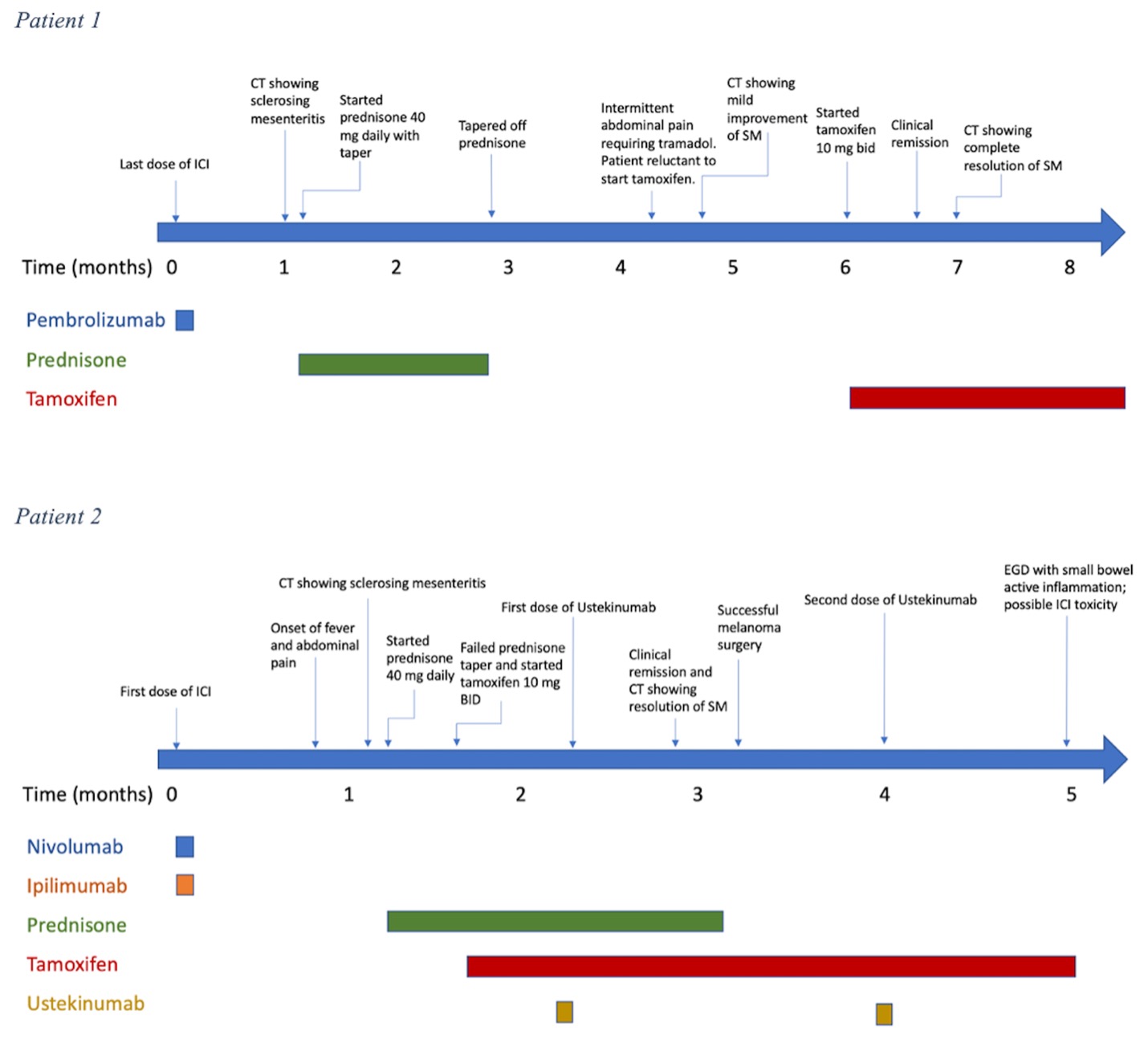
A 66-year-old female with renal cell carcinoma (RCC) on pembrolizumab developed abdominal pain, nausea, and constipation. CT revealed mesenteric lymphadenopathy and fat stranding, indicating SM. Prednisone 40 mg daily slightly improved symptoms and was extended for two months. She eventually tapered off steroids but continued to have intermittent low-grade abdominal pain. Tamoxifen 10 mg twice daily was started and resolved her pain in three weeks, and follow-up CT showed complete resolution of mesenteric inflammation.
Patient 2:
A 47-year-old female with metastatic melanoma on ipilimumab and nivolumab developed fever, night sweats, and abdominal pain. CT showed mesenteric lymphadenopathy and fat stranding, suggesting sclerosing mesenteritis. Prednisone 40 mg daily and tamoxifen 10 mg twice daily were ineffective. Ustekinumab was initiated and improved her symptoms after one dose. Repeat CT after two doses showed complete resolution of mesenteric inflammation, though new liver metastases appeared. She continued ustekinumab for two additional months due to additional development of upper gastrointestinal ICI-related toxicity. She awaits laparoscopic resection of liver metastases and further cancer treatment evaluation.
Discussion: Sclerosing mesenteritis is a rare condition with a multifactorial etiology though recently shown to manifest as a ICI related toxicity. Both patients developed SM after ICI therapy and were unresponsive to corticosteroids. Initiation of tamoxifen and ustekinumab led to clinical and radiological remission within 1 month. Ustekinumab, effective in other inflammatory conditions, demonstrated efficacy in achieving remission when both corticosteroids and tamoxifen failed. These findings suggest that tamoxifen and ustekinumab could be valuable treatment options for ICI-induced SM, contributing to the growing evidence supporting targeted cytokine inhibition in managing this complex condition.
Disclosures:
Abdullah S. Aleem, MD1, Yinghong Wang, MD, PhD2. P2056 - Treatment of Immune Checkpoint Inhibitor Induced Sclerosing Mesenteritis: A Case Series, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Texas at Houston, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Sclerosing mesenteritis (SM) is a rare condition involving nonspecific inflammation and fibrosis of mesenteric fat. While its etiology is unclear, it is associated with autoimmune processes, malignancy, trauma, and and more recently immune mediated toxicity following immune checkpoint inhibitor (ICI) cancer therapy. We report two cases: one achieving remission with tamoxifen after corticosteroid failure, and another improving with ustekinumab, an interleukin-12 and -23 antagonist, after failing both corticosteroids and tamoxifen.
Case Description/Methods: Patient 1:
A 66-year-old female with renal cell carcinoma (RCC) on pembrolizumab developed abdominal pain, nausea, and constipation. CT revealed mesenteric lymphadenopathy and fat stranding, indicating SM. Prednisone 40 mg daily slightly improved symptoms and was extended for two months. She eventually tapered off steroids but continued to have intermittent low-grade abdominal pain. Tamoxifen 10 mg twice daily was started and resolved her pain in three weeks, and follow-up CT showed complete resolution of mesenteric inflammation.
Patient 2:
A 47-year-old female with metastatic melanoma on ipilimumab and nivolumab developed fever, night sweats, and abdominal pain. CT showed mesenteric lymphadenopathy and fat stranding, suggesting sclerosing mesenteritis. Prednisone 40 mg daily and tamoxifen 10 mg twice daily were ineffective. Ustekinumab was initiated and improved her symptoms after one dose. Repeat CT after two doses showed complete resolution of mesenteric inflammation, though new liver metastases appeared. She continued ustekinumab for two additional months due to additional development of upper gastrointestinal ICI-related toxicity. She awaits laparoscopic resection of liver metastases and further cancer treatment evaluation.
Discussion: Sclerosing mesenteritis is a rare condition with a multifactorial etiology though recently shown to manifest as a ICI related toxicity. Both patients developed SM after ICI therapy and were unresponsive to corticosteroids. Initiation of tamoxifen and ustekinumab led to clinical and radiological remission within 1 month. Ustekinumab, effective in other inflammatory conditions, demonstrated efficacy in achieving remission when both corticosteroids and tamoxifen failed. These findings suggest that tamoxifen and ustekinumab could be valuable treatment options for ICI-induced SM, contributing to the growing evidence supporting targeted cytokine inhibition in managing this complex condition.

Figure: Figure 1: Timeline of events for patient 1 and patient 2
Disclosures:
Abdullah Aleem indicated no relevant financial relationships.
Yinghong Wang: AzurRx – Consultant. Ilyapharma – Consultant. IOTA – Consultant. Sorriso – Consultant. Tillotts – Consultant.
Abdullah S. Aleem, MD1, Yinghong Wang, MD, PhD2. P2056 - Treatment of Immune Checkpoint Inhibitor Induced Sclerosing Mesenteritis: A Case Series, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
