Monday Poster Session
Category: Esophagus
P2201 - Monoclonal Antibody Dupilumab Efficiency on Eosinophilic Esophagitis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio
.jpg)
Natasha Mederos, MD
University of Miami Miller School of Medicine at Holy Cross Hospital, FL
Presenting Author(s)
Natasha Mederos, MD, Yaisa Moreno, MD, Nemer Dabage-Forzoli, MD
University of Miami Miller School of Medicine at Holy Cross Hospital, Fort Lauderdale, FL
Introduction: Eosinophilic esophagitis (EoE) is a progressive inflammatory disorder characterized by dysfunction and inflammation within the esophageal tissue. The pathophysiology is attributed to elevated inflammatory infiltrates that cause extensive remodeling, leading to strictures and fibrosis exacerbating dysphagic symptoms. This condition can diminish the quality of life by manifestations of dysphagia and the heightened risk of food impaction.This study aims to evaluate the efficacy of dupilumab at a weekly dose in managing EoE compared to the control group receiving placebo injections.
Methods: Literature search included PubMed, Cochrane Library, Google Scholar, and EMbase. Selection criteria included randomized multicenter double-blinded trials. Pediatric trials were excluded from consideration. Eligibility criteria stipulated that study participants were to be aged over 12 years, diagnosed with EoE by endoscopic biopsy, and unresponsive to PPIs. Exclusion criteria included esophageal strictures that could not be passed with a standard adult upper endoscope, esophageal dilation required at screening, and the use of systemic glucocorticoids for less than three months or swallowed topical glucocorticoids for less than 6 weeks before screening. Primary endpoint was the change in dysphagia questionnaires score from baseline, conducted over a 10-week duration.
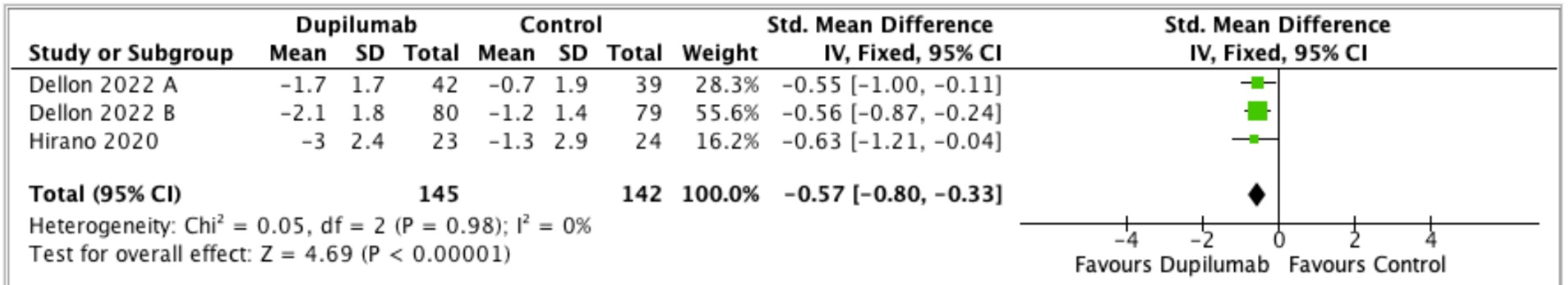
Results: 360 articles were reviewed. Two clinical trials were found and selected; one of these trials included two separate arms with no overlapping patients and was conducted independently. Total of 287 patients were included. Pooled standard mean difference change in dysphagia questionnaires was -0.57 [-0.80,-0.33]. Heterogeneity: Chi2=0.05, df=2 (P=0.98); I2=0% meaning trials were very similar. Overall effect: (Z=4.69, P=< 0.00001; Figure 1) demonstrating improvement on dysphagia scored with weekly dupilumab as compared with placebo.
Discussion: Dupilumab demonstrated a significant improvement in dysphagia compared to the control group. Dupilumab received FDA approval in 2022 for the treatment of eosinophilic esophagitis in individuals aged 12 years and older. Current treatment options include proton pump inhibitors, topical glucocorticoids, Dupilumab, and/or endoscopic dilation, complemented by an empiric elimination diet to prevent flares in EoE. It is crucial to acknowledge, that this meta-analysis faced limitations, including the restricted number of available clinical trials and the comparatively short treatment duration.
Disclosures:
Natasha Mederos, MD, Yaisa Moreno, MD, Nemer Dabage-Forzoli, MD. P2201 - Monoclonal Antibody Dupilumab Efficiency on Eosinophilic Esophagitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
University of Miami Miller School of Medicine at Holy Cross Hospital, Fort Lauderdale, FL
Introduction: Eosinophilic esophagitis (EoE) is a progressive inflammatory disorder characterized by dysfunction and inflammation within the esophageal tissue. The pathophysiology is attributed to elevated inflammatory infiltrates that cause extensive remodeling, leading to strictures and fibrosis exacerbating dysphagic symptoms. This condition can diminish the quality of life by manifestations of dysphagia and the heightened risk of food impaction.This study aims to evaluate the efficacy of dupilumab at a weekly dose in managing EoE compared to the control group receiving placebo injections.
Methods: Literature search included PubMed, Cochrane Library, Google Scholar, and EMbase. Selection criteria included randomized multicenter double-blinded trials. Pediatric trials were excluded from consideration. Eligibility criteria stipulated that study participants were to be aged over 12 years, diagnosed with EoE by endoscopic biopsy, and unresponsive to PPIs. Exclusion criteria included esophageal strictures that could not be passed with a standard adult upper endoscope, esophageal dilation required at screening, and the use of systemic glucocorticoids for less than three months or swallowed topical glucocorticoids for less than 6 weeks before screening. Primary endpoint was the change in dysphagia questionnaires score from baseline, conducted over a 10-week duration.
Results: 360 articles were reviewed. Two clinical trials were found and selected; one of these trials included two separate arms with no overlapping patients and was conducted independently. Total of 287 patients were included. Pooled standard mean difference change in dysphagia questionnaires was -0.57 [-0.80,-0.33]. Heterogeneity: Chi2=0.05, df=2 (P=0.98); I2=0% meaning trials were very similar. Overall effect: (Z=4.69, P=< 0.00001; Figure 1) demonstrating improvement on dysphagia scored with weekly dupilumab as compared with placebo.
Discussion: Dupilumab demonstrated a significant improvement in dysphagia compared to the control group. Dupilumab received FDA approval in 2022 for the treatment of eosinophilic esophagitis in individuals aged 12 years and older. Current treatment options include proton pump inhibitors, topical glucocorticoids, Dupilumab, and/or endoscopic dilation, complemented by an empiric elimination diet to prevent flares in EoE. It is crucial to acknowledge, that this meta-analysis faced limitations, including the restricted number of available clinical trials and the comparatively short treatment duration.

Figure: Figure 1. Standard Mean Difference Between Dupilumab and Control Group Using Dysphagia Questionnaires
Disclosures:
Natasha Mederos indicated no relevant financial relationships.
Yaisa Moreno indicated no relevant financial relationships.
Nemer Dabage-Forzoli indicated no relevant financial relationships.
Natasha Mederos, MD, Yaisa Moreno, MD, Nemer Dabage-Forzoli, MD. P2201 - Monoclonal Antibody Dupilumab Efficiency on Eosinophilic Esophagitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
