Monday Poster Session
Category: Functional Bowel Disease
P2363 - Plecanatide Is Efficacious in Patients With Irritable Bowel Syndrome With Constipation (IBS-C) and Bloating: Evaluation Using Trisymptom Composite Endpoints
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Darren M. Brenner, MD, FACG
Professor of Medicine and Surgery
Northwestern University
Chicago, IL
Presenting Author(s)
Darren M.. Brenner, MD1, Andrea S.. Shin, MD2, Adam P.. Laitman, MD3, David C.. Kunkel, MD4
1Northwestern University, Chicago, IL; 2University of California Los Angeles, Los Angeles, CA; 3Salix Pharmaceuticals, Bridgewater, NJ; 4University of California San Diego, La Jolla, CA
Introduction: Patients with IBS have sensory-related symptoms (eg, abdominal pain, bloating). Bloating is one of the most common symptoms identified in patients with IBS-C, with most reporting moderate/severe bloating. The primary aim of this study was to evaluate the efficacy of plecanatide in an IBS-C population with bloating utilizing a novel trisymptom composite endpoint of cardinal symptoms (ie, pain, bloating, and complete spontaneous bowel movements [CSBMs]) at various thresholds of response.
Methods: Data were pooled and analyzed post hoc from 2 identically designed phase 3 trials. Males/females aged 18-40 y with IBS-C and baseline bloating (score ≥ 1) were treated with plecanatide 3 mg or placebo once daily for 12 wk. CSBM frequency and abdominal pain and bloating intensity (scale range, 0-10 for each) were recorded in a daily diary. Response was defined as simultaneous improvement from baseline in all 3 symptoms (abdominal pain, bloating, and CSBMs/wk) for ≥ 6 of 12 wk of treatment using several composite criteria (ie, ≥ 2-point or ≥ 30% or ≥ 40% improvement in abdominal pain and bloating plus an increase of ≥ 1 or ≥ 2 CSBMs in the same wk for ≥ 6 of 12 wk). Patients were also stratified by baseline bloating intensity (mild [score, 1-5]; moderate/severe [score, 6-10]).
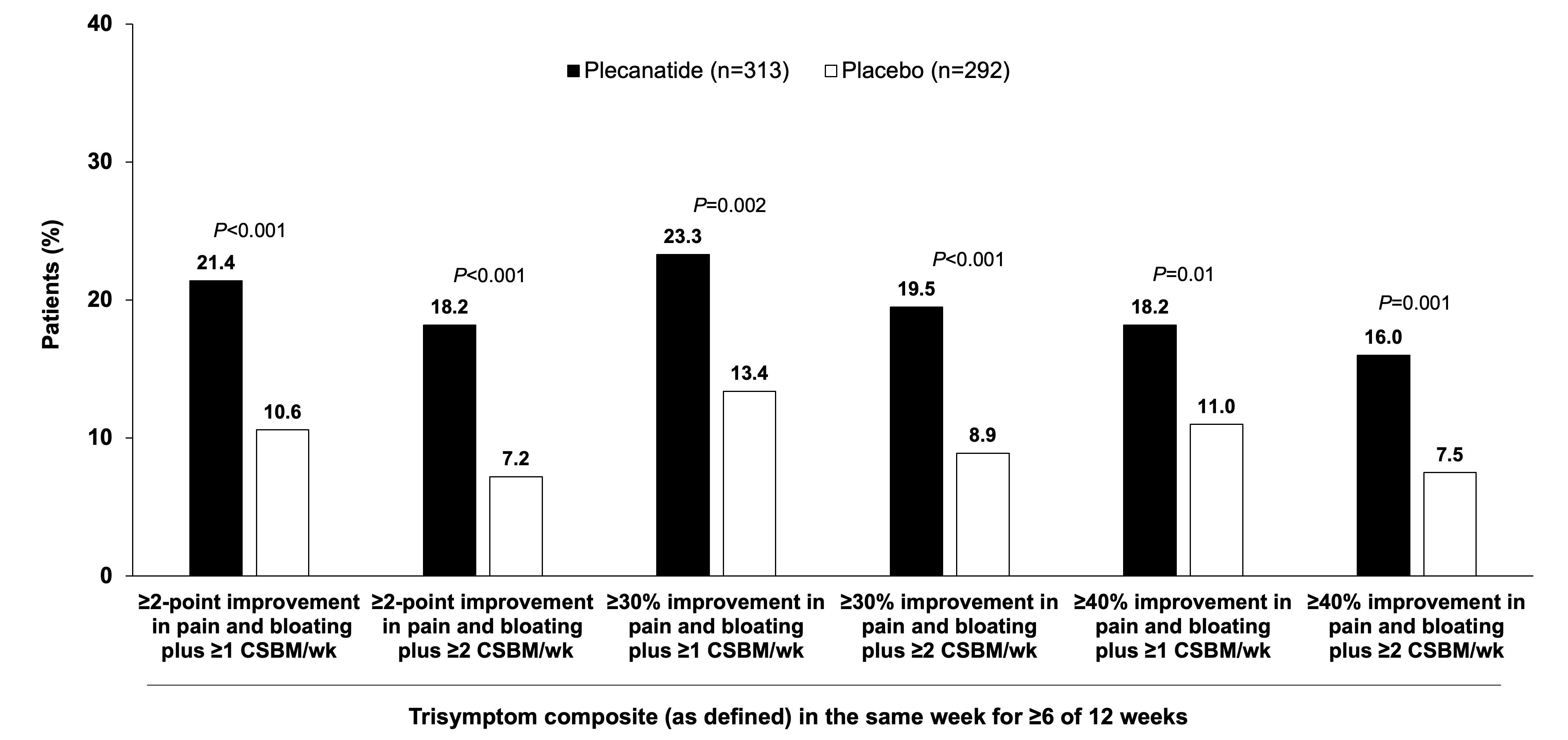
Results: 605 adults with IBS-C and bloating (71.6% female; median age, 31.0 y) were included in the analysis (plecanatide [n = 313]; placebo [n = 292]). Plecanatide and placebo baseline mean symptom scores were 6.2 and 6.4 for abdominal pain and 6.4 and 6.6 for bloating, respectively, and both groups had a mean of 0.2 CSBMs/wk. The majority (71.7%) of patients had moderate/severe bloating. Overall, a significantly greater percentage of patients treated with plecanatide vs placebo were trisymptom composite responders, defined using several stringent thresholds (Figure). When stratified by baseline bloating intensity (mild; moderate/severe), significant improvements favoring plecanatide vs placebo were noted for most of the trisymptom composite endpoints (Table).
Discussion: Plecanatide simultaneously and significantly improved abdominal pain, bloating, and CSBM frequency, using varying thresholds to define trisymptom composite responses. These responses were maintained regardless of baseline bloating intensity. Plecanatide appears effective for treating global and individual sensatory and bowel symptoms characteristic of IBS in patients with IBS-C who reported any bloating at the start of therapy.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Darren M.. Brenner, MD1, Andrea S.. Shin, MD2, Adam P.. Laitman, MD3, David C.. Kunkel, MD4. P2363 - Plecanatide Is Efficacious in Patients With Irritable Bowel Syndrome With Constipation (IBS-C) and Bloating: Evaluation Using Trisymptom Composite Endpoints, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Northwestern University, Chicago, IL; 2University of California Los Angeles, Los Angeles, CA; 3Salix Pharmaceuticals, Bridgewater, NJ; 4University of California San Diego, La Jolla, CA
Introduction: Patients with IBS have sensory-related symptoms (eg, abdominal pain, bloating). Bloating is one of the most common symptoms identified in patients with IBS-C, with most reporting moderate/severe bloating. The primary aim of this study was to evaluate the efficacy of plecanatide in an IBS-C population with bloating utilizing a novel trisymptom composite endpoint of cardinal symptoms (ie, pain, bloating, and complete spontaneous bowel movements [CSBMs]) at various thresholds of response.
Methods: Data were pooled and analyzed post hoc from 2 identically designed phase 3 trials. Males/females aged 18-40 y with IBS-C and baseline bloating (score ≥ 1) were treated with plecanatide 3 mg or placebo once daily for 12 wk. CSBM frequency and abdominal pain and bloating intensity (scale range, 0-10 for each) were recorded in a daily diary. Response was defined as simultaneous improvement from baseline in all 3 symptoms (abdominal pain, bloating, and CSBMs/wk) for ≥ 6 of 12 wk of treatment using several composite criteria (ie, ≥ 2-point or ≥ 30% or ≥ 40% improvement in abdominal pain and bloating plus an increase of ≥ 1 or ≥ 2 CSBMs in the same wk for ≥ 6 of 12 wk). Patients were also stratified by baseline bloating intensity (mild [score, 1-5]; moderate/severe [score, 6-10]).
Results: 605 adults with IBS-C and bloating (71.6% female; median age, 31.0 y) were included in the analysis (plecanatide [n = 313]; placebo [n = 292]). Plecanatide and placebo baseline mean symptom scores were 6.2 and 6.4 for abdominal pain and 6.4 and 6.6 for bloating, respectively, and both groups had a mean of 0.2 CSBMs/wk. The majority (71.7%) of patients had moderate/severe bloating. Overall, a significantly greater percentage of patients treated with plecanatide vs placebo were trisymptom composite responders, defined using several stringent thresholds (Figure). When stratified by baseline bloating intensity (mild; moderate/severe), significant improvements favoring plecanatide vs placebo were noted for most of the trisymptom composite endpoints (Table).
Discussion: Plecanatide simultaneously and significantly improved abdominal pain, bloating, and CSBM frequency, using varying thresholds to define trisymptom composite responses. These responses were maintained regardless of baseline bloating intensity. Plecanatide appears effective for treating global and individual sensatory and bowel symptoms characteristic of IBS in patients with IBS-C who reported any bloating at the start of therapy.

Figure: Figure. IBS-C Trisymptom Composite (Abdominal Pain, Bloating, and CSBM) Responders (Overall Population)
CSBM = complete spontaneous bowel movement; IBS-C = irritable bowel syndrome with constipation.
CSBM = complete spontaneous bowel movement; IBS-C = irritable bowel syndrome with constipation.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Darren Brenner: AbbVie – Consultant, Speaker. Anji Pharmaceuticals – Consultant. Ardelyx – Advisor or Review Panel Member, Consultant, Speaker. Bayer – Consultant. Blueprint Medicines – Advisor or Review Panel Member. CinPhloro – Advisor or Review Panel Member, Consultant. Dr. Reddy's Laboratories – Consultant. Entrinsic Bioscience – Consultant. Gemelli Biotech – Advisor or Review Panel Member, Consultant. Ironwood Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Laborie – Advisor or Review Panel Member. Mahana Therapeutics – Advisor or Review Panel Member, Consultant. Owlstone Medical – Advisor or Review Panel Member, Consultant, Stock Options. Salix Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Vibrant Gastro – Advisor or Review Panel Member, Consultant.
Andrea Shin: Ardelyx Scientific Communications – Advisor or Review Panel Member.
Adam Laitman: Salix Pharmaceuticals – Employee.
David Kunkel: Ardelyx, Evoke Pharma, Mahana Therapeutics, Inc., Pfizer Inc. – Consultant. Regeneron Pharmaceuticals Inc. – Speakers Bureau.
Darren M.. Brenner, MD1, Andrea S.. Shin, MD2, Adam P.. Laitman, MD3, David C.. Kunkel, MD4. P2363 - Plecanatide Is Efficacious in Patients With Irritable Bowel Syndrome With Constipation (IBS-C) and Bloating: Evaluation Using Trisymptom Composite Endpoints, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
