Monday Poster Session
Category: General Endoscopy
P2373 - Effects of Glucagon-Like Peptide-1 Receptor Agonists on Upper Gastrointestinal Endoscopy: A Systematic Review and Meta-Analysis of 84,065 Patients
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Daryl Ramai, MD, MPH, Msc
University of Utah Health
Salt Lake City, UT
Presenting Author(s)
Daryl Ramai, MD, MPH, Msc1, Antonio Facciorusso, MD, PhD2, Jahnvi Dhar, MBBS3, Jayanta Samanta, MBBS3, Saurabh Chandan, MD4, Paraskevas Gkolfakis, MD5, Stefano Crinò, MD6, Marcello Maida, MD7, Andrea Anderloni, MD8, Ivo Boskoski, MD9, Konstantinos Triantafyllou, MD10, Mario Dinis-Ribeiro, MD11, Lorenzo Fuccio, MD12, Marianna Arvanitakis, MD13
1University of Utah Health, Salt Lake City, UT; 2University of Foggia, Foggia, Puglia, Italy; 3Postgraduate Institute of Medical Education and Research, Chandigarh, Chandigarh, India; 4CHI Health Creighton University Medical Center, Omaha, NE; 5Konstantopoulio-Patision General Hospital of Nea Ionia, Athens, Ioannina, Greece; 6University Hospital, Verona, Veneto, Italy; 7University of Enna "Kore", Enna, Lombardia, Italy; 8Fondazione IRCCS Policlinico San Matteo, Pavia, Valle d'Aosta, Italy; 9Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Lazio, Italy; 10Attikon University General Hospital, Athens, Ioannina, Greece; 11University of Porto, Porto, Porto, Portugal; 12University of Bologna, Bologna, Marche, Italy; 13Hôpital Erasme, Brussels, Brussels Hoofdstedelijk Gewest, Belgium
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) were originally developed for the management of type 2 diabetes mellitus (T2DM). Their use has expanded to include the promotion of weight loss in patients with obesity. GLP-1RAs mimic incretins, stimulate insulin secretion after a glucose load, and induce early satiety through delayed gastric emptying. Limited evidence exists regarding the impact of GLP-1RAs on upper endoscopy. Therefore, a meta-analysis was conducted to comprehensively review the available evidence on this subject.
Methods: A systematic bibliographic search was carried out until May 2024. Pooled estimates were analyzed using a random-effects model, with results presented as odds ratios (OR) and 95% confidence intervals (CI). The primary outcome assessed was the rate of retained gastric content (RGC), while secondary outcomes included rates of aborted and repeated procedures, adverse event (AE) rate, and rates of aspiration.
Results: This analysis included 13 studies involving a total of 84,065 patients. Patients receiving GLP-1RA therapy exhibited significantly higher rates of RGC (OR 5.56, 3.35-9.23), a trend that was consistent among diabetic patients (OR 2.60, 2.23-3.02). Adjusted analysis, accounting for variables such as sex, age, body mass index (BMI), diabetes, and other therapies, confirmed the elevated rates of RGC in the GLP-1RA user group (aOR 4.20, 3.42-5.15). Furthermore, rates of aborted and repeated procedures were notably higher in the GLP-1RA user group (OR 5.13, 3.01-8.75, and OR 2.19, 1.43-3.35; respectively). However, no significant differences were found in AE and aspiration rates between the two groups (OR 4.04, 0.63-26.03, and OR 1.75, 0.64-4.77; respectively
Discussion: Our comprehensive analysis indicates that while the use of GLP-1RA results in higher rates of RGC, the actual clinical impact appears to be limited. Additionally, the incidence of adverse events, particularly aspiration, is low and not significantly different between users and non-users of GLP1-RAs. Therefore, there is no strong evidence to support the routine discontinuation of this drug before upper endoscopic procedures. A personalized approach based on specific patient factors such as the reason for GLP-1RA therapy and the type of endoscopic procedure is recommended in this context. Prolonging the duration of fasting for solids might represent the optimal approach in users of GLP1-RAs.
Disclosures:
Daryl Ramai, MD, MPH, Msc1, Antonio Facciorusso, MD, PhD2, Jahnvi Dhar, MBBS3, Jayanta Samanta, MBBS3, Saurabh Chandan, MD4, Paraskevas Gkolfakis, MD5, Stefano Crinò, MD6, Marcello Maida, MD7, Andrea Anderloni, MD8, Ivo Boskoski, MD9, Konstantinos Triantafyllou, MD10, Mario Dinis-Ribeiro, MD11, Lorenzo Fuccio, MD12, Marianna Arvanitakis, MD13. P2373 - Effects of Glucagon-Like Peptide-1 Receptor Agonists on Upper Gastrointestinal Endoscopy: A Systematic Review and Meta-Analysis of 84,065 Patients, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Utah Health, Salt Lake City, UT; 2University of Foggia, Foggia, Puglia, Italy; 3Postgraduate Institute of Medical Education and Research, Chandigarh, Chandigarh, India; 4CHI Health Creighton University Medical Center, Omaha, NE; 5Konstantopoulio-Patision General Hospital of Nea Ionia, Athens, Ioannina, Greece; 6University Hospital, Verona, Veneto, Italy; 7University of Enna "Kore", Enna, Lombardia, Italy; 8Fondazione IRCCS Policlinico San Matteo, Pavia, Valle d'Aosta, Italy; 9Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Lazio, Italy; 10Attikon University General Hospital, Athens, Ioannina, Greece; 11University of Porto, Porto, Porto, Portugal; 12University of Bologna, Bologna, Marche, Italy; 13Hôpital Erasme, Brussels, Brussels Hoofdstedelijk Gewest, Belgium
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) were originally developed for the management of type 2 diabetes mellitus (T2DM). Their use has expanded to include the promotion of weight loss in patients with obesity. GLP-1RAs mimic incretins, stimulate insulin secretion after a glucose load, and induce early satiety through delayed gastric emptying. Limited evidence exists regarding the impact of GLP-1RAs on upper endoscopy. Therefore, a meta-analysis was conducted to comprehensively review the available evidence on this subject.
Methods: A systematic bibliographic search was carried out until May 2024. Pooled estimates were analyzed using a random-effects model, with results presented as odds ratios (OR) and 95% confidence intervals (CI). The primary outcome assessed was the rate of retained gastric content (RGC), while secondary outcomes included rates of aborted and repeated procedures, adverse event (AE) rate, and rates of aspiration.
Results: This analysis included 13 studies involving a total of 84,065 patients. Patients receiving GLP-1RA therapy exhibited significantly higher rates of RGC (OR 5.56, 3.35-9.23), a trend that was consistent among diabetic patients (OR 2.60, 2.23-3.02). Adjusted analysis, accounting for variables such as sex, age, body mass index (BMI), diabetes, and other therapies, confirmed the elevated rates of RGC in the GLP-1RA user group (aOR 4.20, 3.42-5.15). Furthermore, rates of aborted and repeated procedures were notably higher in the GLP-1RA user group (OR 5.13, 3.01-8.75, and OR 2.19, 1.43-3.35; respectively). However, no significant differences were found in AE and aspiration rates between the two groups (OR 4.04, 0.63-26.03, and OR 1.75, 0.64-4.77; respectively
Discussion: Our comprehensive analysis indicates that while the use of GLP-1RA results in higher rates of RGC, the actual clinical impact appears to be limited. Additionally, the incidence of adverse events, particularly aspiration, is low and not significantly different between users and non-users of GLP1-RAs. Therefore, there is no strong evidence to support the routine discontinuation of this drug before upper endoscopic procedures. A personalized approach based on specific patient factors such as the reason for GLP-1RA therapy and the type of endoscopic procedure is recommended in this context. Prolonging the duration of fasting for solids might represent the optimal approach in users of GLP1-RAs.

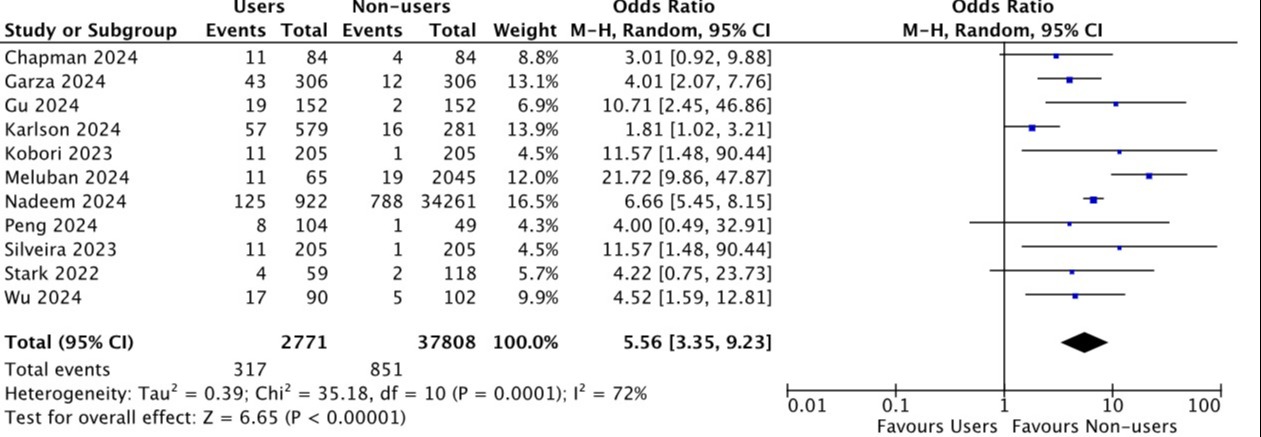
Figure: Forest plot comparing the rate of retained gastric content . The rate of retained gastric content was significantly higher in the group of patients using GLP-1 RA (OR 5.56, 3.35-9.23; p<0.001).
Disclosures:
Daryl Ramai indicated no relevant financial relationships.
Antonio Facciorusso indicated no relevant financial relationships.
Jahnvi Dhar indicated no relevant financial relationships.
Jayanta Samanta indicated no relevant financial relationships.
Saurabh Chandan indicated no relevant financial relationships.
Paraskevas Gkolfakis indicated no relevant financial relationships.
Stefano Crinò indicated no relevant financial relationships.
Marcello Maida indicated no relevant financial relationships.
Andrea Anderloni indicated no relevant financial relationships.
Ivo Boskoski: Apollo Endosurgery – Consultant. Boston Scientific – Consultant. Cook Medical – Consultant. Endo Tools Therapeutics S.A. – Consultant. ERBE – Consultant. Microtech – Consultant. Nitinotes – Consultant. Pentax – Consultant.
Konstantinos Triantafyllou indicated no relevant financial relationships.
Mario Dinis-Ribeiro indicated no relevant financial relationships.
Lorenzo Fuccio indicated no relevant financial relationships.
Marianna Arvanitakis indicated no relevant financial relationships.
Daryl Ramai, MD, MPH, Msc1, Antonio Facciorusso, MD, PhD2, Jahnvi Dhar, MBBS3, Jayanta Samanta, MBBS3, Saurabh Chandan, MD4, Paraskevas Gkolfakis, MD5, Stefano Crinò, MD6, Marcello Maida, MD7, Andrea Anderloni, MD8, Ivo Boskoski, MD9, Konstantinos Triantafyllou, MD10, Mario Dinis-Ribeiro, MD11, Lorenzo Fuccio, MD12, Marianna Arvanitakis, MD13. P2373 - Effects of Glucagon-Like Peptide-1 Receptor Agonists on Upper Gastrointestinal Endoscopy: A Systematic Review and Meta-Analysis of 84,065 Patients, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
