Monday Poster Session
Category: IBD
P2571 - Real-World Effectiveness and Onset of Action of Vedolizumab as a First-Line Biologic in Biologic-Naïve Patients With Ulcerative Colitis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- TR
Timothy E. Ritter, MD
GI Alliance
Southlake, TX
Presenting Author(s)
Precious A. Anyanwu, PharmD, PhD1, Lucinda J. Van Anglen, BS, PharmD1, Chiahung Chou, PhD2, Marie Sanchirico, MD, PhD2, Timothy E. Ritter, MD3
1Healix Infusion Therapy, LLC, Sugar Land, TX; 2Takeda Pharmaceuticals, USA, Inc., Cambridge, MA; 3GI Alliance, Southlake, TX
Introduction: In a prior analysis of data from this real-world study we found that anti-tumor necrosis factor α drugs can be used as second-line biologic treatments in patients with ulcerative colitis (UC) who had previously received vedolizumab as a first-line biologic without concerns about their effectiveness.1 Here we report further data on the effectiveness and onset of action of vedolizumab as a first-line biologic in biologic-naïve patients with UC.
Methods: This retrospective observational study included biologic-naïve adults with mild to severe UC who were treated at a large, multicenter, private gastroenterology practice. Patients who received vedolizumab as a first-line biologic between January 2018 and May 2020 were identified through electronic medical records. Key exploratory endpoints were the proportion of patients with clinical remission (partial Mayo score < 2) and the proportion of patients with corticosteroid-free remission (clinical remission and no longer receiving corticosteroids) at 3, 6, 9, and 12 months after vedolizumab treatment initiation. The onset of action was assessed using partial Mayo scores (decrease of ≥ 1 point in rectal bleeding or ≥ 1 point in stool frequency from baseline) at weeks 2 and 6.
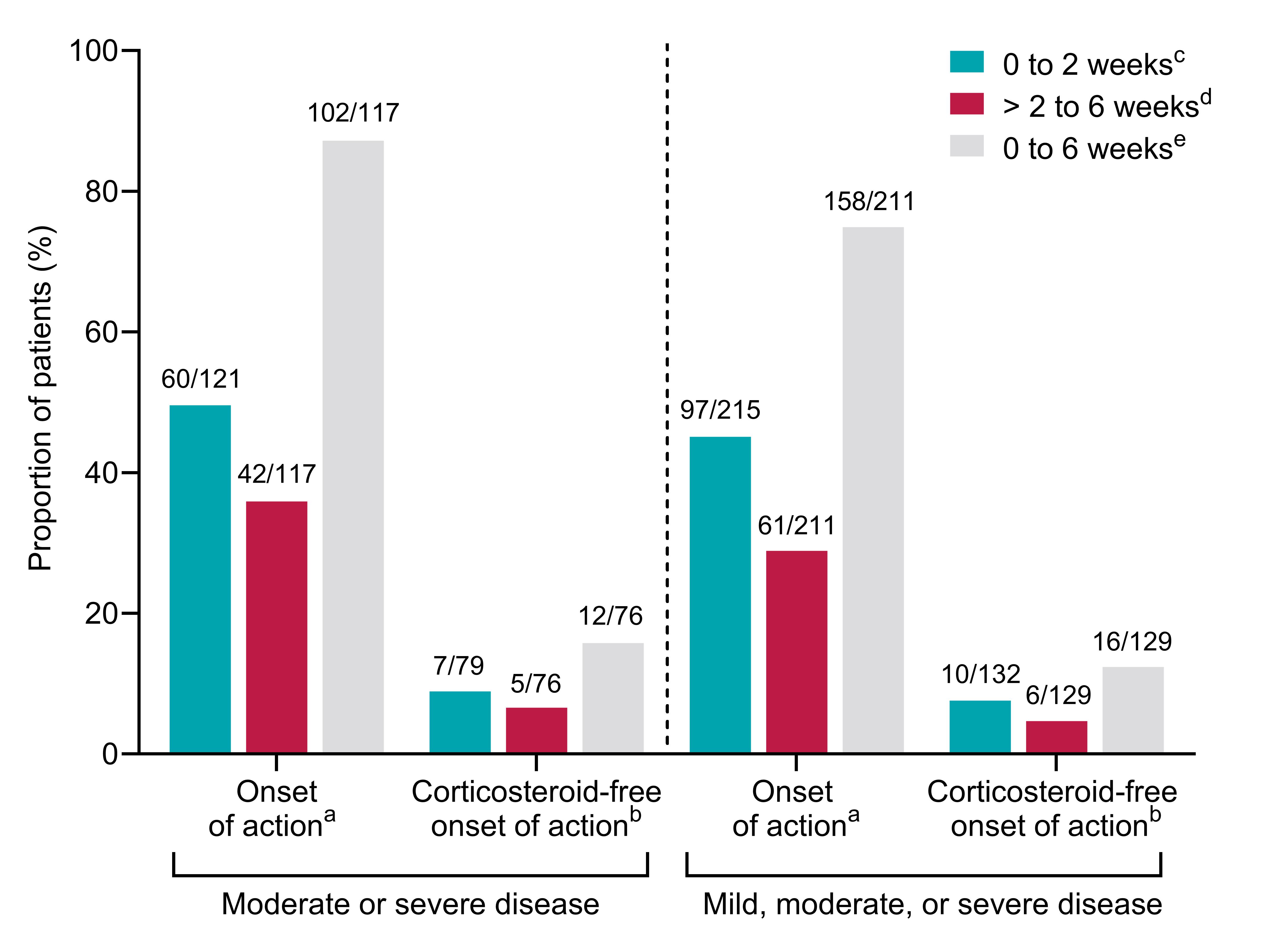
Results: In total, 260 patients received vedolizumab as a first-line biologic. Patients had a mean age of 42.6 years at baseline and 45.0% were female. All patients began maintenance therapy with intravenous vedolizumab 300 mg once every 8 weeks and 15.4% required dose escalation within 12 months. At time of vedolizumab initiation, 60.8% of patients were receiving a concurrent corticosteroid. The proportions of patients who had clinical remission or corticosteroid-free remission are shown in Table 1. At 12 months, 149/188 patients (79.3%) who remained on vedolizumab had clinical remission. Of 109 patients who were receiving a corticosteroid at baseline and remained on vedolizumab at 12 months, 52.3% were corticosteroid-free and had remission. Onset of action was observed by week 6 in 87.2% of patients with moderate or severe disease; 49.6% had a response to vedolizumab within 2 weeks of initiation (Figure 1).
Discussion: These real-world data show that vedolizumab as a first-line biologic produced remission in a high percentage of biologic-naïve patients with UC. Importantly, the data indicate that the onset of action of vedolizumab typically manifests in less than 6 weeks for most patients.
1. Anyanwu PA, et al. Gastroenterology. 2024;166(5):S1147-1148.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Precious A. Anyanwu, PharmD, PhD1, Lucinda J. Van Anglen, BS, PharmD1, Chiahung Chou, PhD2, Marie Sanchirico, MD, PhD2, Timothy E. Ritter, MD3. P2571 - Real-World Effectiveness and Onset of Action of Vedolizumab as a First-Line Biologic in Biologic-Naïve Patients With Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Healix Infusion Therapy, LLC, Sugar Land, TX; 2Takeda Pharmaceuticals, USA, Inc., Cambridge, MA; 3GI Alliance, Southlake, TX
Introduction: In a prior analysis of data from this real-world study we found that anti-tumor necrosis factor α drugs can be used as second-line biologic treatments in patients with ulcerative colitis (UC) who had previously received vedolizumab as a first-line biologic without concerns about their effectiveness.1 Here we report further data on the effectiveness and onset of action of vedolizumab as a first-line biologic in biologic-naïve patients with UC.
Methods: This retrospective observational study included biologic-naïve adults with mild to severe UC who were treated at a large, multicenter, private gastroenterology practice. Patients who received vedolizumab as a first-line biologic between January 2018 and May 2020 were identified through electronic medical records. Key exploratory endpoints were the proportion of patients with clinical remission (partial Mayo score < 2) and the proportion of patients with corticosteroid-free remission (clinical remission and no longer receiving corticosteroids) at 3, 6, 9, and 12 months after vedolizumab treatment initiation. The onset of action was assessed using partial Mayo scores (decrease of ≥ 1 point in rectal bleeding or ≥ 1 point in stool frequency from baseline) at weeks 2 and 6.
Results: In total, 260 patients received vedolizumab as a first-line biologic. Patients had a mean age of 42.6 years at baseline and 45.0% were female. All patients began maintenance therapy with intravenous vedolizumab 300 mg once every 8 weeks and 15.4% required dose escalation within 12 months. At time of vedolizumab initiation, 60.8% of patients were receiving a concurrent corticosteroid. The proportions of patients who had clinical remission or corticosteroid-free remission are shown in Table 1. At 12 months, 149/188 patients (79.3%) who remained on vedolizumab had clinical remission. Of 109 patients who were receiving a corticosteroid at baseline and remained on vedolizumab at 12 months, 52.3% were corticosteroid-free and had remission. Onset of action was observed by week 6 in 87.2% of patients with moderate or severe disease; 49.6% had a response to vedolizumab within 2 weeks of initiation (Figure 1).
Discussion: These real-world data show that vedolizumab as a first-line biologic produced remission in a high percentage of biologic-naïve patients with UC. Importantly, the data indicate that the onset of action of vedolizumab typically manifests in less than 6 weeks for most patients.
1. Anyanwu PA, et al. Gastroenterology. 2024;166(5):S1147-1148.

Figure: Figure 1. Onset of action and corticosteroid-free onset of action in patients with UC who received vedolizumab as a first-line biologic
UC, ulcerative colitis.
Patients with remission at baseline are excluded from analysis.
a Partial Mayo score decrease of ≥ 1 point in rectal bleeding or ≥ 1 point in stool frequency from baseline assessed at week 2 or week 6. b Patients who are not receiving a concurrent corticosteroid at each time point. c Patients with onset of action at week 2. Denominator excludes patients who discontinued or were lost to follow-up. d Patients without onset of action at week 2 and with onset of action at week 6. Denominator excludes patients who discontinued or were lost to follow-up. e All patients with onset of action by week 6. Denominator excludes patients who discontinued or were lost to follow-up at week 6.
UC, ulcerative colitis.
Patients with remission at baseline are excluded from analysis.
a Partial Mayo score decrease of ≥ 1 point in rectal bleeding or ≥ 1 point in stool frequency from baseline assessed at week 2 or week 6. b Patients who are not receiving a concurrent corticosteroid at each time point. c Patients with onset of action at week 2. Denominator excludes patients who discontinued or were lost to follow-up. d Patients without onset of action at week 2 and with onset of action at week 6. Denominator excludes patients who discontinued or were lost to follow-up. e All patients with onset of action by week 6. Denominator excludes patients who discontinued or were lost to follow-up at week 6.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Precious Anyanwu indicated no relevant financial relationships.
Lucinda Van Anglen: Cumberland Pharmaceuticals – Grant/Research Support. Ferring Pharmaceuticals – Grant/Research Support. Novartis Pharmaceuticals – Grant/Research Support. Takeda Pharmaceuticals – Grant/Research Support.
Chiahung Chou: Takeda Pharmaceuticals U.S.A., Inc. – Employee, Stock Options.
Marie Sanchirico: Takeda Pharmaceuticals U.S.A., Inc. – Employee, Stock Options.
Timothy Ritter: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Ardelyx – Advisory Committee/Board Member. Arena Pharmaceuticals – Advisory Committee/Board Member. Boehringer Ingelheim – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celgene – Advisory Committee/Board Member. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Ferring – Advisory Committee/Board Member. Ferring/Rebiotix – has served on a Data Adjudication Committee. Genentech (Roche) – Advisory Committee/Board Member. Intercept – Advisory Committee/Board Member. Iterative Scopes – Advisory Committee/Board Member, holds shares in Iterative Scopes. Janssen – Advisory Committee/Board Member, Speakers Bureau. Nestlé/Seres – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Prometheus – Advisory Committee/Board Member. Sanofi – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member, Speakers Bureau.
Precious A. Anyanwu, PharmD, PhD1, Lucinda J. Van Anglen, BS, PharmD1, Chiahung Chou, PhD2, Marie Sanchirico, MD, PhD2, Timothy E. Ritter, MD3. P2571 - Real-World Effectiveness and Onset of Action of Vedolizumab as a First-Line Biologic in Biologic-Naïve Patients With Ulcerative Colitis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
