Monday Poster Session
Category: IBD
P2629 - Clinical Validation of a Novel Software Tool to Guide the Intravenous-to-Subcutaneous Switching of Infliximab in Patients With Inflammatory Bowel Diseases
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- YH
Yannick Hoffert, PharmD
Katholieke Universiteit Leuven
Leuven, Vlaams-Brabant, Belgium
Presenting Author(s)
Award: Presidential Poster Award
Yannick Hoffert, PharmD1, Zhigang Wang, MSc, PharmD1, Mathurin Fumery, MD, PhD2, Maria Nachury, MD3, Maeva Bazoge, MSc4, Anthony Buisson, MD, PhD4, Erwin Dreesen, PharmD; PhD1
1Katholieke Universiteit Leuven, Leuven, Vlaams-Brabant, Belgium; 2CHU Amiens and Péritox, Picardie Jules Verne University, Amiens, Amiens, Picardie, France; 3Lille University and Hospital, Lille, Nord-Pas-de-Calais, France; 4Université Clermont Auvergne, CHU Clemront-Ferrand, Clermont-Ferrand, Auvergne, France
Introduction: Therapeutic drug monitoring (TDM) of intravenous (IV) infliximab is increasingly practiced to improve therapeutic outcomes in patients with inflammatory bowel diseases (IBD). Recently, a subcutaneous (SC) formulation of infliximab was approved for maintenance treatment of IBD.
In the present work, we introduce a software tool to guide infliximab dosing by predicting infliximab concentrations after switching to the SC formulation.
Methods: An infliximab population pharmacokinetics (popPK) model, published in the European Public Assessment Report (EMA/CHMP/548703/2019), was used in this study. A software tool was developed using the Shiny package (v1.7.4) in R (v4.3.0). Bayesian forecasting was implemented using mapbayr (v0.10.0), which was used to estimate individual PK parameters through maximum a posteriori estimation.
External validation data were obtained from the prospective, multicenter REMSWITCH trial [1]. For each patient, a pre-switch infliximab trough concentration (TC) was used to predict a post-switch TC. Predicted and observed post-switch TCs were compared, and absolute and relative prediction errors (PE) were calculated. Relapse was defined as an increase in fecal calprotectin >=150 mg/g from baseline.
Results: A total of 76 patients contributed one pre/post-switch TC pair each. Three patients had detectable antibodies towards infliximab pre-switch. Three other patients were cotreated with methotrexate pre-switch.
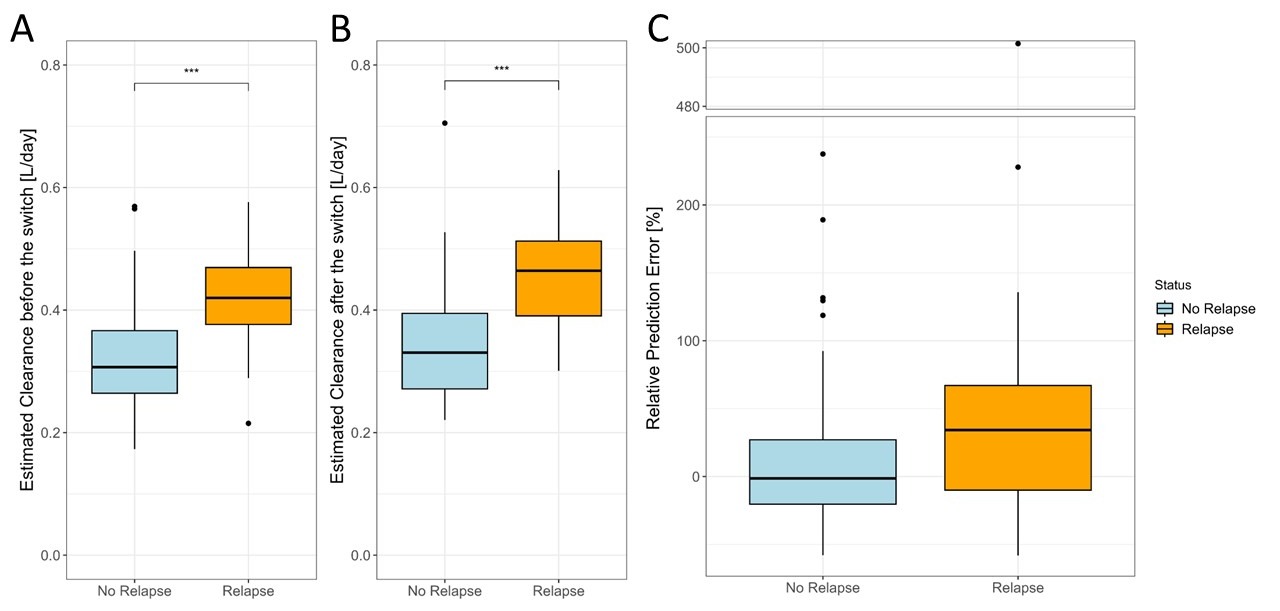
The estimated median clearance using TCs pre-switch was 0.31 L/day for the relapse-free cohort, and 0.42 L/day for the relapse cohort (p < 0.001) (Figure 1A). The estimated median clearance using TCs post-witch was 0.33 L/day for the relapse-free cohort, and 0.46 L/day for the relapse cohort (p < 0.001) (Figure 1B). Furthermore, there is a tendency towards higher positive prediction errors for the relapse group (Figure 1C).
Discussion: The clinically validated software tool (https://lpmx.shinyapps.io/infliximab/) allows interactive simulations and individual predictions to guide infliximab dosing while switching formulations. Predictions for patients without relapse were unbiased yet precision is likely compromised by variable SC bioavailability or time-varying PK. Significantly higher estimated clearance in the relapsed cohort resulted in an overprediction of TC, likely driven by increased disease activity and thus lower-than-expected infliximab observations. These findings serve as an early predictor for relapse, warranting closer monitoring of these patients.
Disclosures:
Yannick Hoffert, PharmD1, Zhigang Wang, MSc, PharmD1, Mathurin Fumery, MD, PhD2, Maria Nachury, MD3, Maeva Bazoge, MSc4, Anthony Buisson, MD, PhD4, Erwin Dreesen, PharmD; PhD1. P2629 - Clinical Validation of a Novel Software Tool to Guide the Intravenous-to-Subcutaneous Switching of Infliximab in Patients With Inflammatory Bowel Diseases, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Yannick Hoffert, PharmD1, Zhigang Wang, MSc, PharmD1, Mathurin Fumery, MD, PhD2, Maria Nachury, MD3, Maeva Bazoge, MSc4, Anthony Buisson, MD, PhD4, Erwin Dreesen, PharmD; PhD1
1Katholieke Universiteit Leuven, Leuven, Vlaams-Brabant, Belgium; 2CHU Amiens and Péritox, Picardie Jules Verne University, Amiens, Amiens, Picardie, France; 3Lille University and Hospital, Lille, Nord-Pas-de-Calais, France; 4Université Clermont Auvergne, CHU Clemront-Ferrand, Clermont-Ferrand, Auvergne, France
Introduction: Therapeutic drug monitoring (TDM) of intravenous (IV) infliximab is increasingly practiced to improve therapeutic outcomes in patients with inflammatory bowel diseases (IBD). Recently, a subcutaneous (SC) formulation of infliximab was approved for maintenance treatment of IBD.
In the present work, we introduce a software tool to guide infliximab dosing by predicting infliximab concentrations after switching to the SC formulation.
Methods: An infliximab population pharmacokinetics (popPK) model, published in the European Public Assessment Report (EMA/CHMP/548703/2019), was used in this study. A software tool was developed using the Shiny package (v1.7.4) in R (v4.3.0). Bayesian forecasting was implemented using mapbayr (v0.10.0), which was used to estimate individual PK parameters through maximum a posteriori estimation.
External validation data were obtained from the prospective, multicenter REMSWITCH trial [1]. For each patient, a pre-switch infliximab trough concentration (TC) was used to predict a post-switch TC. Predicted and observed post-switch TCs were compared, and absolute and relative prediction errors (PE) were calculated. Relapse was defined as an increase in fecal calprotectin >=150 mg/g from baseline.
Results: A total of 76 patients contributed one pre/post-switch TC pair each. Three patients had detectable antibodies towards infliximab pre-switch. Three other patients were cotreated with methotrexate pre-switch.
The estimated median clearance using TCs pre-switch was 0.31 L/day for the relapse-free cohort, and 0.42 L/day for the relapse cohort (p < 0.001) (Figure 1A). The estimated median clearance using TCs post-witch was 0.33 L/day for the relapse-free cohort, and 0.46 L/day for the relapse cohort (p < 0.001) (Figure 1B). Furthermore, there is a tendency towards higher positive prediction errors for the relapse group (Figure 1C).
Discussion: The clinically validated software tool (https://lpmx.shinyapps.io/infliximab/) allows interactive simulations and individual predictions to guide infliximab dosing while switching formulations. Predictions for patients without relapse were unbiased yet precision is likely compromised by variable SC bioavailability or time-varying PK. Significantly higher estimated clearance in the relapsed cohort resulted in an overprediction of TC, likely driven by increased disease activity and thus lower-than-expected infliximab observations. These findings serve as an early predictor for relapse, warranting closer monitoring of these patients.

Figure: Figure 1: Boxplots showing the distribution of the estimated clearance before the switch (Panel A), after the switch (Panel B), and the relative prediction error of the predicted infliximab exposure (Panel C), stratified by relapse and relapse-free cohort. The prediction error was calculated as; (Predicted – Observed) /Observed*100%. All p-value calculations were performed on a two-sided t-test. ***p <0.001.
Disclosures:
Yannick Hoffert indicated no relevant financial relationships.
Zhigang Wang: Celltrion – Sponsorship of travel and accomendation cost for ACG2024 conference.
Mathurin Fumery indicated no relevant financial relationships.
Maria Nachury indicated no relevant financial relationships.
Maeva Bazoge indicated no relevant financial relationships.
Anthony Buisson: Abbvie – Consultant. Abbvie – Lecture fees. Ferring – Lecture fees. Hospira – Lecture fees. MSD – Lecture fees. Takeda – Consultant. Takeda – Lecture fees. Vifor Pharma – Lecture fees.
Erwin Dreesen: Alimentiv – Consultant. argenx – Consultant. Celltrion – Speakers Bureau. Galapagos – Speakers Bureau. Janssen – Grant/Research Support. Prometheus – Grant/Research Support. Sandoz – Grant/Research Support.
Yannick Hoffert, PharmD1, Zhigang Wang, MSc, PharmD1, Mathurin Fumery, MD, PhD2, Maria Nachury, MD3, Maeva Bazoge, MSc4, Anthony Buisson, MD, PhD4, Erwin Dreesen, PharmD; PhD1. P2629 - Clinical Validation of a Novel Software Tool to Guide the Intravenous-to-Subcutaneous Switching of Infliximab in Patients With Inflammatory Bowel Diseases, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


