Monday Poster Session
Category: IBD
P2682 - Impact of Baseline Disease Duration on Ozanimod Efficacy in Patients With Moderately to Severely Active Ulcerative Colitis: A Post Hoc Analysis of the Phase 3 True North Study
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Gary Lichtenstein, MD, FACG
Perelman Center for Advanced Medicine, University of Pennsylvania
Philadelphia, PA
Presenting Author(s)
Gary R. Lichtenstein, MD, FACG1, Geert R. D'Haens, MD, PhD2, Alissa Walsh, MD3, Sarah C. Glover, DO4, Dana J. Lukin, MD5, Benjamin L. Cohen, MD6, Garrett Lawlor, MD7, Melissa Rosen, MD7, Hsiuanlin Wu, MS7, Mark T. Osterman, MD7, Anjali Jain, PhD7, Ailsa Hart, PhD8
1Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia, PA; 2Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands; 3Translational Gastroenterology Unit, Oxford University Hospital, Oxford, England, United Kingdom; 4Tulane University School of Medicine, New Orleans, LA; 5Jill Roberts Center for Inflammatory Bowel Disease, New York Presbyterian Hospital-Weill Cornell Medicine, New York, NY; 6Cleveland Clinic Foundation, Cleveland, OH; 7Bristol Myers Squibb, Princeton, NJ; 8St. Mark’s Hospital and Imperial College, London, England, United Kingdom
Introduction: Ozanimod (OZA) is approved for patients (pts) with moderately to severely active ulcerative colitis (UC) based on results from the pivotal phase 3 True North (TN) trial.
Methods: This analysis evaluated the impact of disease duration at TN baseline (BL) on OZA efficacy during the TN induction period (IP). In the 10-wk TN IP, pts were randomized to receive in a double-blind fashion either placebo (PBO) or OZA in Cohort 1 or open-label OZA in Cohort 2. All Cohort 1 pts were included in this analysis and stratified by disease duration at TN BL; subgroups included pts with < 2, 2 to < 5, 5 to < 10, or ≥10 y since UC diagnosis. Symptomatic remission and response were assessed through Week (W) 10 within these subgroups using PBO-adjusted difference in proportions; treatment differences and P values for comparisons between OZA and PBO were based on the Cochran-Mantel-Haenszel test, stratified by corticosteroid use at screening and prior anti–tumor necrosis factor use. Clinical response and remission at W10 were also analyzed. A stepwise logistic regression model was used to adjust for potential BL pt characteristic confounders.
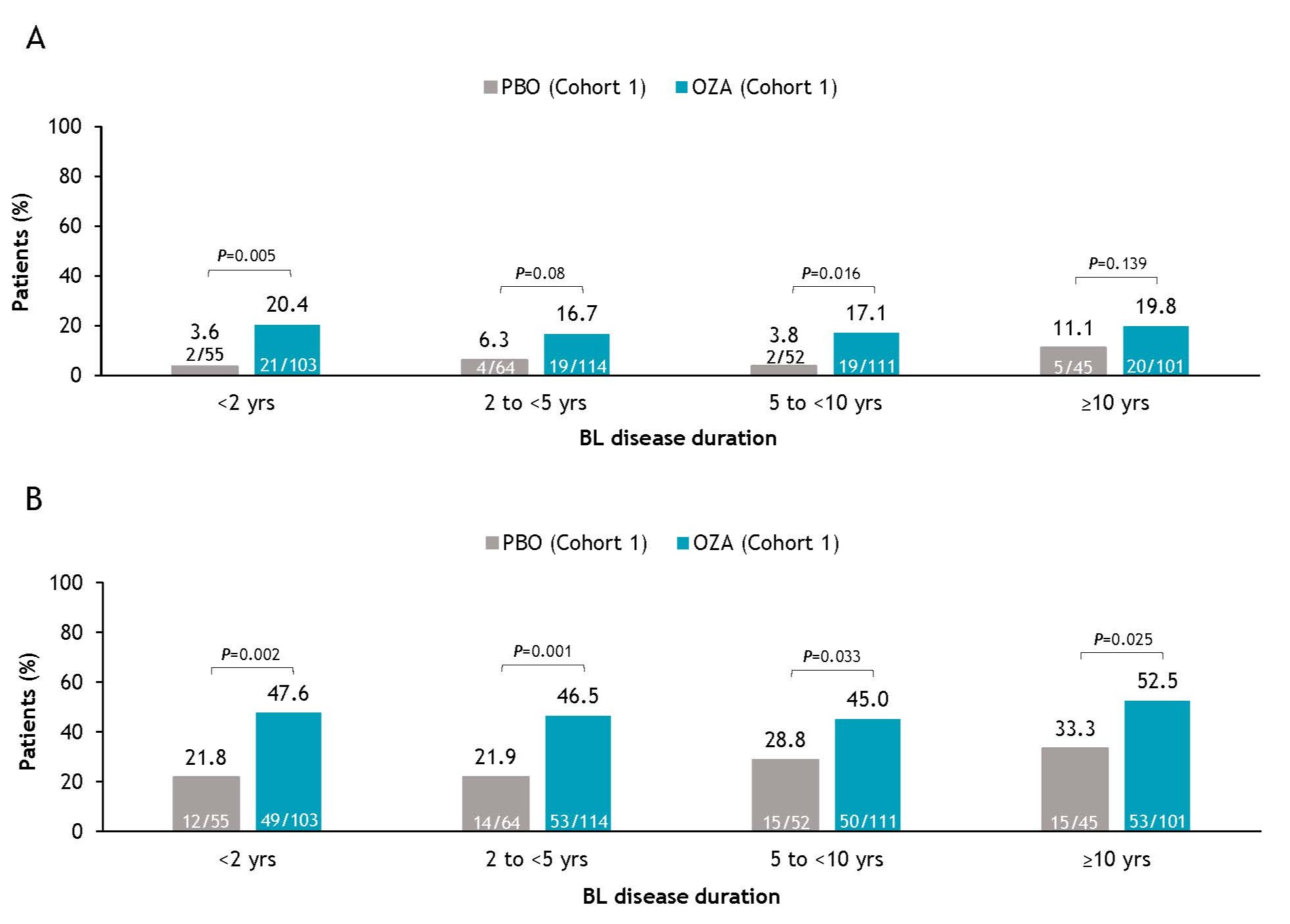
Results: A total of 645 pts were included in Cohort 1 of the TN IP (PBO, N=216; OZA, N=429). At TN BL, 158 pts had a disease duration of < 2 y (PBO, n=55; OZA, n=103), 178 had 2 to < 5 y (PBO, n=64; OZA, n=114), 163 had 5 to < 10 y (PBO, n=52; OZA, n=111), and 146 had ≥10 y (PBO, n=45; OZA, n=101). BL pt characteristics were generally similar in all subgroups of the OZA and PBO treatment arms. As expected, more pts with longer disease duration had prior exposure to biologic therapies. Pts with disease duration < 2 y achieved greater symptomatic clinical response by W4 (P< 0.001) compared to other subgroups, but differences were similar across other subgroups by W10; symptomatic remission rates were also generally similar across subgroups by W10. The proportions of pts achieving clinical remission and response after 10 wk of OZA treatment were comparable, regardless of TN BL disease duration (Figure). Finally, a logistic regression model demonstrated that BL disease duration did not affect OZA efficacy.
Discussion: OZA efficacy at the end of induction was not affected by duration of disease in pts with UC. These results suggest that OZA is an optimal oral therapeutic option for newly diagnosed pts and for those with long-standing UC disease.
Disclosures:
Gary R. Lichtenstein, MD, FACG1, Geert R. D'Haens, MD, PhD2, Alissa Walsh, MD3, Sarah C. Glover, DO4, Dana J. Lukin, MD5, Benjamin L. Cohen, MD6, Garrett Lawlor, MD7, Melissa Rosen, MD7, Hsiuanlin Wu, MS7, Mark T. Osterman, MD7, Anjali Jain, PhD7, Ailsa Hart, PhD8. P2682 - Impact of Baseline Disease Duration on Ozanimod Efficacy in Patients With Moderately to Severely Active Ulcerative Colitis: A Post Hoc Analysis of the Phase 3 True North Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Perelman Center for Advanced Medicine, University of Pennsylvania, Philadelphia, PA; 2Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands; 3Translational Gastroenterology Unit, Oxford University Hospital, Oxford, England, United Kingdom; 4Tulane University School of Medicine, New Orleans, LA; 5Jill Roberts Center for Inflammatory Bowel Disease, New York Presbyterian Hospital-Weill Cornell Medicine, New York, NY; 6Cleveland Clinic Foundation, Cleveland, OH; 7Bristol Myers Squibb, Princeton, NJ; 8St. Mark’s Hospital and Imperial College, London, England, United Kingdom
Introduction: Ozanimod (OZA) is approved for patients (pts) with moderately to severely active ulcerative colitis (UC) based on results from the pivotal phase 3 True North (TN) trial.
Methods: This analysis evaluated the impact of disease duration at TN baseline (BL) on OZA efficacy during the TN induction period (IP). In the 10-wk TN IP, pts were randomized to receive in a double-blind fashion either placebo (PBO) or OZA in Cohort 1 or open-label OZA in Cohort 2. All Cohort 1 pts were included in this analysis and stratified by disease duration at TN BL; subgroups included pts with < 2, 2 to < 5, 5 to < 10, or ≥10 y since UC diagnosis. Symptomatic remission and response were assessed through Week (W) 10 within these subgroups using PBO-adjusted difference in proportions; treatment differences and P values for comparisons between OZA and PBO were based on the Cochran-Mantel-Haenszel test, stratified by corticosteroid use at screening and prior anti–tumor necrosis factor use. Clinical response and remission at W10 were also analyzed. A stepwise logistic regression model was used to adjust for potential BL pt characteristic confounders.
Results: A total of 645 pts were included in Cohort 1 of the TN IP (PBO, N=216; OZA, N=429). At TN BL, 158 pts had a disease duration of < 2 y (PBO, n=55; OZA, n=103), 178 had 2 to < 5 y (PBO, n=64; OZA, n=114), 163 had 5 to < 10 y (PBO, n=52; OZA, n=111), and 146 had ≥10 y (PBO, n=45; OZA, n=101). BL pt characteristics were generally similar in all subgroups of the OZA and PBO treatment arms. As expected, more pts with longer disease duration had prior exposure to biologic therapies. Pts with disease duration < 2 y achieved greater symptomatic clinical response by W4 (P< 0.001) compared to other subgroups, but differences were similar across other subgroups by W10; symptomatic remission rates were also generally similar across subgroups by W10. The proportions of pts achieving clinical remission and response after 10 wk of OZA treatment were comparable, regardless of TN BL disease duration (Figure). Finally, a logistic regression model demonstrated that BL disease duration did not affect OZA efficacy.
Discussion: OZA efficacy at the end of induction was not affected by duration of disease in pts with UC. These results suggest that OZA is an optimal oral therapeutic option for newly diagnosed pts and for those with long-standing UC disease.

Figure: Figure . Clinical (A) remissiona and (B) responseb at W10 in all pts in Cohort 1 of the TN IP by BL disease duration. All P values are nominal. aClinical remission: RBS = 0, SFS ≤1 point (and a decrease of ≥1 point from BL SFS), and MES ≤1 point. bClinical response: decrease from BL in the 3-component Mayo score (sum of RBS, SFS, and MES) of ≥2 points and ≥35% and a reduction of ≥1 point in RBS or absolute RBS of ≤1 point. BL, baseline; IP, induction period; MES, Mayo endoscopy subscore; OZA, ozanimod; PBO, placebo; pt, patient; RBS, rectal bleeding subscore; SFS, stool frequency subscore; TN, True North; W, Week.
Disclosures:
Gary R. Lichtenstein: AbbVie – Consultant. American College of Gastroenterology – honoraria. American Gastroenterological Association – CME. American Regent – Consultant, Honorarium. Celgene – Consultant, Grant/Research Support. Cellceutix – Consultant. Chemed – CME. Eli Lilly – Advisory Committee/Board Member, Consultant. Endo – Consultant. Ferring – Consultant. Gastroenterology & Hepatology – Gastro-Hep Communication, Editor- Honorarium. Gilead – Consultant. IMEDEX – CME. Ironwood – CME. Janssen – Consultant, Grant/Research Support. MedEd Consultants – Consultant. Merck – Consultant, Honorarium. Morphic Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support. Professional Communications Inc. – Royalties. Prometheus Laboratories – Consultant. Romark – Consultant, honoraria. Salix/Valeant – Consultant. Sandoz – Consultant. Shire – Consultant. SLACK Inc. – Royalties. Springer Science and Business Media – Honorarium. Takeda – Consultant, Grant/Research Support. UCB – Consultant, Grant/Research Support. University of Kentucky – CME. UpToDate – honoraria. Vindico – CME. Virgo – Consultant, Stock Options.
Geert D'Haens: AbbVie – Advisor or Review Panel Member, Speakers Bureau. Agomab Therapeutics – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. Allergan – Advisor or Review Panel Member. Alphabiomics – Advisor or Review Panel Member. AstraZeneca – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Speakers Bureau. Ferring – Advisor or Review Panel Member. Galapagos – Advisor or Review Panel Member, Speakers Bureau. GlaxoSmithKline – Advisor or Review Panel Member. Immunic – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Seres – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau. Tillotts – Advisor or Review Panel Member, Speakers Bureau. Ventyx – Advisor or Review Panel Member.
Alissa Walsh: AbbVie – Consultant, Grant/Research Support. Bühlmann Laboratories – Consultant, Grant/Research Support. Eli Lilly – Consultant, Grant/Research Support. Galapagos – Consultant, Grant/Research Support. GlaxoSmithKline – Consultant, Grant/Research Support. Janssen – Consultant, Grant/Research Support. Pfizer Inc – Consultant, Grant/Research Support. Takeda – Consultant, Grant/Research Support. Vifor – Consultant, Grant/Research Support.
Sarah C. Glover indicated no relevant financial relationships.
Dana J. Lukin: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. AltruBio – Consultant. Boehringer Ingelheim – Consultant, Grant/Research Support. Bristol Myers Squibb – Consultant. Eli Lilly – Consultant. Fresenius Kabi – Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Magellan Health – Consultant. Palatin Technologies – Consultant. Pfizer – Consultant. Prometheus Laboratories – Consultant. PSI – Consultant. Takeda – Consultant, Grant/Research Support.
Benjamin L. Cohen: Abbvie – Consultant, support and/or funding, Speakers Bureau. Bristol Myers Squibb – support and/or funding. Celgene – support and/or funding. Emmes – Consultant, Speakers Bureau. Janssen – Consultant, support and/or funding, Speakers Bureau. Pfizer – support and/or funding. Takeda – Consultant, Speakers Bureau. Target RWE – Consultant, Speakers Bureau.
Garrett Lawlor: Bristol Myers Squibb – Employee.
Melissa Rosen: Bristol Myers Squibb – Employee.
Hsiuanlin Wu: Bristol Myers Squibb – Employee.
Mark T. Osterman: Bristol Myers Squibb – Employee.
Anjali Jain: Bristol Myers Squibb – Employee.
Ailsa Hart: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Atlantic Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Dr. Falk Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Genentech – Global Steering Committee. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. MSD – Advisory Committee/Board Member, Consultant, Speakers Bureau. Napp Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pharmacosmos – Advisory Committee/Board Member, Consultant, Speakers Bureau. Shire – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Gary R. Lichtenstein, MD, FACG1, Geert R. D'Haens, MD, PhD2, Alissa Walsh, MD3, Sarah C. Glover, DO4, Dana J. Lukin, MD5, Benjamin L. Cohen, MD6, Garrett Lawlor, MD7, Melissa Rosen, MD7, Hsiuanlin Wu, MS7, Mark T. Osterman, MD7, Anjali Jain, PhD7, Ailsa Hart, PhD8. P2682 - Impact of Baseline Disease Duration on Ozanimod Efficacy in Patients With Moderately to Severely Active Ulcerative Colitis: A Post Hoc Analysis of the Phase 3 True North Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
