Monday Poster Session
Category: IBD
P2688 - Risk of Thromboembolic Events Among Inflammatory Bowel Disease Patients on Janus Kinase Inhibitors
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- SG
Sanya Goswami, MD
SUNY Downstate Health Sciences University
Brooklyn, NY
Presenting Author(s)
Sanya Goswami, MD1, Rahul Raiker, MD2, Farah Monzur, MD, FACG3
1SUNY Downstate Health Sciences University, Brooklyn, NY; 2George Washington University School of Medicine and Health Sciences, Washington, DC; 3Renaissance School of Medicine at Stony Brook University, Stony Brook, NY
Introduction: Janus kinase inhibitors (JAKi) have emerged as a treatment option by targeting key cytokines involved in IBD. Venous thromboembolism (VTE) is a well-known complication in patients with ulcerative colitis (UC) and Crohn’s disease (CD) due to the complex interplay of the inflammatory state and coagulation cascade. Previous studies have found an increased risk of VTE in rheumatoid arthritis patients on JAKi but limited data exists describing this risk in IBD patients. Therefore, the goal of this study is to examine the 1-year risk of various thromboembolic events among IBD patients on JAKi.
Methods: A retrospective cohort analysis was conducted using TriNetX, a multi-center database of ~110 million patients across 80 healthcare organizations. Adults with a diagnosis of IBD were identified from 2018-2023. This cohort was then split into those who initiated JAKi treatment and controls who never started on JAKi treatment. A 1:1 propensity score matching was then conducted, controlling for comorbidities and demographics, to calculate adjusted hazard ratios (aHR) with 95% CI for 1-year risk of thromboembolic events between the cohorts. aHR was estimated using the Cox proportional hazard model. Thromboembolic events was a composite outcome defined, as a first time instance of cerebral infarction, transient ischemic attack, any arterial embolism/thrombosis, or any venous embolism/thrombosis. A subgroup analysis was also conducted for UC patients on tofacitinib or upadacitinib and CD patients on upadacitinib only. Patients with outcomes prior to the index event were excluded.
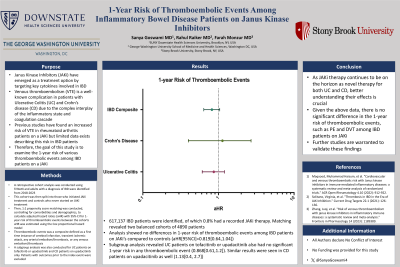
Results: 617,137 IBD patients were identified, of which 0.8% had a recorded JAKi therapy. Matching revealed two balanced cohorts of 4890 patients. Analysis showed no differences in 1-year risk of thromboembolic events among IBD patients on JAKi’s compared to controls (aHR[95%CI]=0.819[0.64,1.04]). Subgroup analysis revealed UC patients on tofacitinib or upadacitinib also had no significant 1-year risk in any thromboembolic event (0.868[0.61,1.2]). Similar results were seen in CD patients on upadacitinib as well (1.13[0.4, 2.7]).
Discussion: As JAK inhibitors are a novel therapy for both UC and CD, better understanding of their side effects is crucial. Given the above data, there is no significant difference in the 1-year risk of thromboembolic events among IBD patients on JAKi’s. Further long-term studies are warranted to validate these findings.
Disclosures:
Sanya Goswami, MD1, Rahul Raiker, MD2, Farah Monzur, MD, FACG3. P2688 - Risk of Thromboembolic Events Among Inflammatory Bowel Disease Patients on Janus Kinase Inhibitors, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1SUNY Downstate Health Sciences University, Brooklyn, NY; 2George Washington University School of Medicine and Health Sciences, Washington, DC; 3Renaissance School of Medicine at Stony Brook University, Stony Brook, NY
Introduction: Janus kinase inhibitors (JAKi) have emerged as a treatment option by targeting key cytokines involved in IBD. Venous thromboembolism (VTE) is a well-known complication in patients with ulcerative colitis (UC) and Crohn’s disease (CD) due to the complex interplay of the inflammatory state and coagulation cascade. Previous studies have found an increased risk of VTE in rheumatoid arthritis patients on JAKi but limited data exists describing this risk in IBD patients. Therefore, the goal of this study is to examine the 1-year risk of various thromboembolic events among IBD patients on JAKi.
Methods: A retrospective cohort analysis was conducted using TriNetX, a multi-center database of ~110 million patients across 80 healthcare organizations. Adults with a diagnosis of IBD were identified from 2018-2023. This cohort was then split into those who initiated JAKi treatment and controls who never started on JAKi treatment. A 1:1 propensity score matching was then conducted, controlling for comorbidities and demographics, to calculate adjusted hazard ratios (aHR) with 95% CI for 1-year risk of thromboembolic events between the cohorts. aHR was estimated using the Cox proportional hazard model. Thromboembolic events was a composite outcome defined, as a first time instance of cerebral infarction, transient ischemic attack, any arterial embolism/thrombosis, or any venous embolism/thrombosis. A subgroup analysis was also conducted for UC patients on tofacitinib or upadacitinib and CD patients on upadacitinib only. Patients with outcomes prior to the index event were excluded.
Results: 617,137 IBD patients were identified, of which 0.8% had a recorded JAKi therapy. Matching revealed two balanced cohorts of 4890 patients. Analysis showed no differences in 1-year risk of thromboembolic events among IBD patients on JAKi’s compared to controls (aHR[95%CI]=0.819[0.64,1.04]). Subgroup analysis revealed UC patients on tofacitinib or upadacitinib also had no significant 1-year risk in any thromboembolic event (0.868[0.61,1.2]). Similar results were seen in CD patients on upadacitinib as well (1.13[0.4, 2.7]).
Discussion: As JAK inhibitors are a novel therapy for both UC and CD, better understanding of their side effects is crucial. Given the above data, there is no significant difference in the 1-year risk of thromboembolic events among IBD patients on JAKi’s. Further long-term studies are warranted to validate these findings.
Disclosures:
Sanya Goswami indicated no relevant financial relationships.
Rahul Raiker indicated no relevant financial relationships.
Farah Monzur: Gather-Ed – Consultant, Speakers Bureau. Medtronic (Covidien) – Consultant. Prometheus – Consultant.
Sanya Goswami, MD1, Rahul Raiker, MD2, Farah Monzur, MD, FACG3. P2688 - Risk of Thromboembolic Events Among Inflammatory Bowel Disease Patients on Janus Kinase Inhibitors, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
