Tuesday Poster Session
Category: IBD
P4276 - Impact of GLP-1RA on MACE in Patients With IBD
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- MS
Michael Saadeh, MD
University Hospitals Cleveland Medical Center, Case Western Reserve University
Cleveland, OH
Presenting Author(s)
Award: Presidential Poster Award
Michael Saadeh, MD1, Apoorva K. Chandar, MBBS, MPH2, Sadeer Al-Kindi, MD3, Vu Q. Nguyen, MD, MS4, Revital Gorodeski Baskin, MD5, Jeffry Katz, MD4, Fabio Cominelli, MD, PhD4, Emad Mansoor, MD4
1University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH; 2Case Western Reserve University School of Medicine, Cleveland, OH; 3Houston Methodist Hospital, Houston, TX; 4Digestive Health Institute, University Hospitals Cleveland Medical Center, Cleveland, OH; 5University Hospitals Cleveland Medical Center, Cleveland, OH
Introduction: Patients with chronic inflammatory disorders are at an increased risk of major adverse cardiovascular events (MACE). Inflammatory bowel disease (IBD) is associated with increased risk of MACE due to chronic systemic inflammation, lipid dysfunction, and endothelial dysfunction. Recent trials have shown that Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RA), an anti-diabetes and anti-obesity drug class, have a beneficial impact on cardiovascular outcomes in patients with metabolic disorders. We hypothesized GLP-1RA will reduce MACE in patients with IBD.
Methods: Using TriNetX, a large healthcare database comprising over 110 million patients in the United States, we identified cohorts of patients with IBD on one or more IBD medications using ICD-10 codes. We categorized these IBD patients into 2 subgroups based on their exposure to GLP-1RA. Cases were IBD patients on a GLP-1RA, while controls were IBD patients without exposure to GLP-1RA. The two cohorts were propensity matched for demographics, comorbidities, cardioprotective medications, and IBD medications. The outcome of interest was MACE, defined as cerebrovascular accident, acute heart failure exacerbation, and acute myocardial infarction 6 months to 5 years after the index event of GLP-1RA use. We stratified our analysis by the inclusion or exclusion of prior MACE. Chi-square and t-tests were used for significance testing.
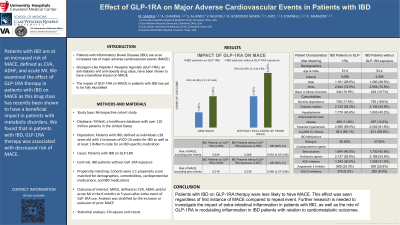
Results: There were 4,804 cases and 146,872 controls. After propensity matching, there were 4,059 cases and controls who were evenly matched. After excluding prior MACE, 3,659 cases and 3,557 controls remained. When compared to patients with IBD and no exposure to GLP-1RA, those with IBD on GLP-1RA were less likely to have new onset MACE (OR 0.49, 95% CI 0.37-0.66). Furthermore, this beneficial effect persisted even when including patients with prior MACE (OR 0.55, 95% CI 0.45-0.68).
Discussion: Patients with IBD on GLP-1RA therapy were less likely to have MACE. Further research is needed to investigate the impact of extra-intestinal inflammation in patients with IBD, as well as the role of GLP-1RA in modulating inflammation in IBD patients with relation to cardiometabolic outcomes.

Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Michael Saadeh, MD1, Apoorva K. Chandar, MBBS, MPH2, Sadeer Al-Kindi, MD3, Vu Q. Nguyen, MD, MS4, Revital Gorodeski Baskin, MD5, Jeffry Katz, MD4, Fabio Cominelli, MD, PhD4, Emad Mansoor, MD4. P4276 - Impact of GLP-1RA on MACE in Patients With IBD, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Michael Saadeh, MD1, Apoorva K. Chandar, MBBS, MPH2, Sadeer Al-Kindi, MD3, Vu Q. Nguyen, MD, MS4, Revital Gorodeski Baskin, MD5, Jeffry Katz, MD4, Fabio Cominelli, MD, PhD4, Emad Mansoor, MD4
1University Hospitals Cleveland Medical Center, Case Western Reserve University, Cleveland, OH; 2Case Western Reserve University School of Medicine, Cleveland, OH; 3Houston Methodist Hospital, Houston, TX; 4Digestive Health Institute, University Hospitals Cleveland Medical Center, Cleveland, OH; 5University Hospitals Cleveland Medical Center, Cleveland, OH
Introduction: Patients with chronic inflammatory disorders are at an increased risk of major adverse cardiovascular events (MACE). Inflammatory bowel disease (IBD) is associated with increased risk of MACE due to chronic systemic inflammation, lipid dysfunction, and endothelial dysfunction. Recent trials have shown that Glucagon-Like Peptide-1 Receptor Agonists (GLP-1RA), an anti-diabetes and anti-obesity drug class, have a beneficial impact on cardiovascular outcomes in patients with metabolic disorders. We hypothesized GLP-1RA will reduce MACE in patients with IBD.
Methods: Using TriNetX, a large healthcare database comprising over 110 million patients in the United States, we identified cohorts of patients with IBD on one or more IBD medications using ICD-10 codes. We categorized these IBD patients into 2 subgroups based on their exposure to GLP-1RA. Cases were IBD patients on a GLP-1RA, while controls were IBD patients without exposure to GLP-1RA. The two cohorts were propensity matched for demographics, comorbidities, cardioprotective medications, and IBD medications. The outcome of interest was MACE, defined as cerebrovascular accident, acute heart failure exacerbation, and acute myocardial infarction 6 months to 5 years after the index event of GLP-1RA use. We stratified our analysis by the inclusion or exclusion of prior MACE. Chi-square and t-tests were used for significance testing.
Results: There were 4,804 cases and 146,872 controls. After propensity matching, there were 4,059 cases and controls who were evenly matched. After excluding prior MACE, 3,659 cases and 3,557 controls remained. When compared to patients with IBD and no exposure to GLP-1RA, those with IBD on GLP-1RA were less likely to have new onset MACE (OR 0.49, 95% CI 0.37-0.66). Furthermore, this beneficial effect persisted even when including patients with prior MACE (OR 0.55, 95% CI 0.45-0.68).
Discussion: Patients with IBD on GLP-1RA therapy were less likely to have MACE. Further research is needed to investigate the impact of extra-intestinal inflammation in patients with IBD, as well as the role of GLP-1RA in modulating inflammation in IBD patients with relation to cardiometabolic outcomes.

Figure: Figure 1 depicts the risk of MACE in IBD patients on GLP-1RA compared to IBD patients without exposure to GLP-1RA. The beneficial impact persists even when including patients with prior MACE.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Michael Saadeh indicated no relevant financial relationships.
Apoorva Chandar indicated no relevant financial relationships.
Sadeer Al-Kindi indicated no relevant financial relationships.
Vu Nguyen: AbbVie – Speakers Bureau. Eli Lilly – Speakers Bureau.
Revital Gorodeski Baskin indicated no relevant financial relationships.
Jeffry Katz indicated no relevant financial relationships.
Fabio Cominelli indicated no relevant financial relationships.
Emad Mansoor: Lilly – Speakers Bureau. Takeda – Speakers Bureau.
Michael Saadeh, MD1, Apoorva K. Chandar, MBBS, MPH2, Sadeer Al-Kindi, MD3, Vu Q. Nguyen, MD, MS4, Revital Gorodeski Baskin, MD5, Jeffry Katz, MD4, Fabio Cominelli, MD, PhD4, Emad Mansoor, MD4. P4276 - Impact of GLP-1RA on MACE in Patients With IBD, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


