Tuesday Poster Session
Category: IBD
P4288 - Psychometric Evaluation of the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) in Adults With Moderate to Severe Crohn’s Disease
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- AV
Aisha Vadhariya
Eli Lilly and Company
Indianapolis, IN
Presenting Author(s)
Miguel D Regueiro, MD1, Sylvia Su, 2, Aisha Vadhariya, 2, Xian Zhou, 3, Frederick Durand, 2, Larissa Stassek, 4, Ariane Kawata, 4, Claudine Clucas, 4, Vipul Jairath, MBChB5
1Cleveland Clinic, Cleveland, OH; 2Eli Lilly and Company, Indianapolis, IN; 3Syneos Health, Morrisville, NC; 4Evidera, Bethesda, MD; 5Western University, London, ON, Canada
Introduction: This study provides evidence on the measurement properties of the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-Fatigue) and determines thresholds for meaningful improvement in patients with moderately to severely active Crohn's disease (CD).
Methods: Pooled data from VIVID-1, a phase 3, multicenter, randomized, placebo- and active-controlled study of mirikizumab, were used to evaluate the reliability, validity, and responsiveness of the FACIT-Fatigue at Weeks 0 (Baseline), 12, and 52. Other assessments completed in the trial were used for these analyses, including the: Patient Global Rating of Severity (PGRS), Patient Global Impression of Change (PGIC), Inflammatory Bowel Disease Questionnaire (IBDQ), Crohn’s Disease Activity Index (CDAI), and 36-Item Short Form Health Survey (SF-36). An anchor-based approach was used to estimate the FACIT-Fatigue total score that would constitute meaningful change. PGRS and PGIC were used as primary anchors.
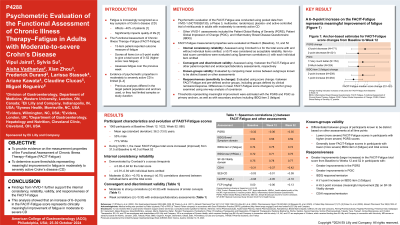
Results: Analyses included 1065 adults (mean age 36.2±13.0 years). The mean FACIT-Fatigue total score improved during the study (Baseline: 31.5; Week 12: 37.1; Week 52: 40.0). The measure demonstrated excellent internal consistency and reliability across visits (Cronbach’s alpha: 0.92–0.94); correlations between individual items and the total score were moderate (0.30≤ r < 0.70) to large (r ≥0.70). Across visits, the FACIT-Fatigue showed moderate-to-large correlations with the PGRS; IBDQ Bowel Function domain, item 2 (fatigue), and total score; CDAI score; and SF-36 Vitality domain score. Correlations with clinical measures were weak (r < 0.30), as hypothesized. FACIT-Fatigue could discriminate between participant groups known to differ based on PGRS, IBDQ total score, and IBDQ item 2. FACIT-Fatigue was able to detect change, as total score improvements from baseline to weeks 12 and 52 were statistically significantly different between most PGRS change groups and PGIC groups, and between groups based on IBDQ response/remission, IBDQ item 2, SF-36 Vitality domain, and clinical response/remission by CDAI. Anchor-based analyses suggested that a 6–9-point increase in the FACIT-Fatigue total score would represent meaningful improvement.
Discussion: The findings expand upon previous evidence supporting the use of the FACIT-Fatigue as a reliable and valid measure in adults with moderately to severely active CD, with a clinically meaningful improvement threshold range of 6-9 points.
Disclosures:
Miguel D Regueiro, MD1, Sylvia Su, 2, Aisha Vadhariya, 2, Xian Zhou, 3, Frederick Durand, 2, Larissa Stassek, 4, Ariane Kawata, 4, Claudine Clucas, 4, Vipul Jairath, MBChB5. P4288 - Psychometric Evaluation of the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) in Adults With Moderate to Severe Crohn’s Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Cleveland Clinic, Cleveland, OH; 2Eli Lilly and Company, Indianapolis, IN; 3Syneos Health, Morrisville, NC; 4Evidera, Bethesda, MD; 5Western University, London, ON, Canada
Introduction: This study provides evidence on the measurement properties of the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-Fatigue) and determines thresholds for meaningful improvement in patients with moderately to severely active Crohn's disease (CD).
Methods: Pooled data from VIVID-1, a phase 3, multicenter, randomized, placebo- and active-controlled study of mirikizumab, were used to evaluate the reliability, validity, and responsiveness of the FACIT-Fatigue at Weeks 0 (Baseline), 12, and 52. Other assessments completed in the trial were used for these analyses, including the: Patient Global Rating of Severity (PGRS), Patient Global Impression of Change (PGIC), Inflammatory Bowel Disease Questionnaire (IBDQ), Crohn’s Disease Activity Index (CDAI), and 36-Item Short Form Health Survey (SF-36). An anchor-based approach was used to estimate the FACIT-Fatigue total score that would constitute meaningful change. PGRS and PGIC were used as primary anchors.
Results: Analyses included 1065 adults (mean age 36.2±13.0 years). The mean FACIT-Fatigue total score improved during the study (Baseline: 31.5; Week 12: 37.1; Week 52: 40.0). The measure demonstrated excellent internal consistency and reliability across visits (Cronbach’s alpha: 0.92–0.94); correlations between individual items and the total score were moderate (0.30≤ r < 0.70) to large (r ≥0.70). Across visits, the FACIT-Fatigue showed moderate-to-large correlations with the PGRS; IBDQ Bowel Function domain, item 2 (fatigue), and total score; CDAI score; and SF-36 Vitality domain score. Correlations with clinical measures were weak (r < 0.30), as hypothesized. FACIT-Fatigue could discriminate between participant groups known to differ based on PGRS, IBDQ total score, and IBDQ item 2. FACIT-Fatigue was able to detect change, as total score improvements from baseline to weeks 12 and 52 were statistically significantly different between most PGRS change groups and PGIC groups, and between groups based on IBDQ response/remission, IBDQ item 2, SF-36 Vitality domain, and clinical response/remission by CDAI. Anchor-based analyses suggested that a 6–9-point increase in the FACIT-Fatigue total score would represent meaningful improvement.
Discussion: The findings expand upon previous evidence supporting the use of the FACIT-Fatigue as a reliable and valid measure in adults with moderately to severely active CD, with a clinically meaningful improvement threshold range of 6-9 points.
Disclosures:
Miguel D Regueiro: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Sylvia Su: Eli Lilly and Company – Employee, Stock Options.
Aisha Vadhariya: Eli Lilly and Company – Employee, Stock Options.
Xian Zhou: Syneos Health – Employee.
Frederick Durand: Eli Lilly and Company – Employee, Stock Options.
Larissa Stassek: Evidera – Employee.
Ariane Kawata: Evidera – Employee.
Claudine Clucas: Evidera – Employee.
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Employee, Grant/Research Support, Speakers Bureau. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Advisory Committee/Board Member, Consultant. Enthera – Advisory Committee/Board Member, Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Advisory Committee/Board Member, Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Advisory Committee/Board Member, Consultant. JAMP – Advisory Committee/Board Member, Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. London Health Sciences Centre – Employee. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Advisory Committee/Board Member, Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. SCOPE – Advisory Committee/Board Member, Consultant. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Synedgen – Advisory Committee/Board Member, Consultant. Takeda – Consultant, Grant/Research Support, Speakers Bureau. TD Securities – Advisory Committee/Board Member, Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Miguel D Regueiro, MD1, Sylvia Su, 2, Aisha Vadhariya, 2, Xian Zhou, 3, Frederick Durand, 2, Larissa Stassek, 4, Ariane Kawata, 4, Claudine Clucas, 4, Vipul Jairath, MBChB5. P4288 - Psychometric Evaluation of the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) in Adults With Moderate to Severe Crohn’s Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
