Tuesday Poster Session
Category: IBD
P4321 - Fecal Calprotectin Completion Patterns in a Mixed Academic-Community Health System
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- MS
Matthew B. Stanton, MD
Northwestern University
Chicago, IL
Presenting Author(s)
Matthew B. Stanton, MD1, Saihej P. Basra, 2, Cenfu Wei, MS1, Stephen B. Hanauer, MD, FACG3, Parambir S. Dulai, MD2
1Northwestern University, Chicago, IL; 2Feinberg School of Medicine, Northwestern University, Chicago, IL; 3Northwestern Medicine, Chicago, IL
Introduction: Societal guidelines recommend fecal calprotectin (FC) based monitoring of disease activity in inflammatory bowel disease (IBD). Claims data suggest low FC utilization at the population level, and small single center cohort studies suggest variation in FC completion at the patient level. We aimed to characterize FC utilization in a large cohort study and identify factors associated with FC completion to guide effective implementation.
Methods: Using an integrated health system and enterprise data warehouse, we conducted a multi-center mixed academic-community cohort study. FC orders between 2012-2024 were identified, and attributes related to patient demographics, ordering providers, and lab orders including completion rates and time to completion were characterized. Temporal analyses were performed to characterize changes in ordering and completion patterns in relation to key events (COVID pandemic, AGA biomarker guideline release, change in health system lab testing with 48-hour FC turnaround time). Univariate and multivariate logistic regression analyses were performed to identify predictors of FC completion.
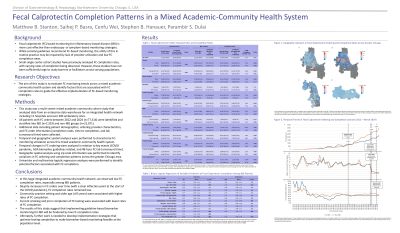
Results: We identified 28,424 patients (n=7,353 IBD) with 77,314 FC orders (n=33,720 IBD FC orders). Overall FC completion rates were low (37%) and they were lowest among IBD patients (34%). Temporal analyses identified a significant increase in FC ordering during the COVID pandemic, with no change in completion rates. The release of the AGA biomarker guidelines and change in internal lab turnaround time for test results had no impact on FC ordering or completion rates. Multivariate analyses identified that male patients (adjusted OR (aOR) 1.05, 95% Confidence Interval (CI) 1.01-1.10, P=.027), patients over the age of 65 (aOR 1.15, 95% CI 1.06-1.25, P< .001), those with private insurance (aOR 1.07, 95% CI 1.00-1.15, P=.040), non-white patients (aOR 1.13, 95% CI 1.06-1.20, P< .001), and those cared for in a community setting (aOR 2.07, 95% CI 1.97-2.17, P< .001), were significantly more likely to complete FC testing.
Discussion: In this large cohort study that spanned academic and community locations, we observed low completion rates for FC. Despite a significant increase in ordering during the COVID pandemic, completion rates did not improve. We identified several factors associated with increased FC completion and these data suggest that community-based integration of biomarker-based monitoring is feasible but requires effective strategies to optimize successful implementation.
Disclosures:
Matthew B. Stanton, MD1, Saihej P. Basra, 2, Cenfu Wei, MS1, Stephen B. Hanauer, MD, FACG3, Parambir S. Dulai, MD2. P4321 - Fecal Calprotectin Completion Patterns in a Mixed Academic-Community Health System, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Northwestern University, Chicago, IL; 2Feinberg School of Medicine, Northwestern University, Chicago, IL; 3Northwestern Medicine, Chicago, IL
Introduction: Societal guidelines recommend fecal calprotectin (FC) based monitoring of disease activity in inflammatory bowel disease (IBD). Claims data suggest low FC utilization at the population level, and small single center cohort studies suggest variation in FC completion at the patient level. We aimed to characterize FC utilization in a large cohort study and identify factors associated with FC completion to guide effective implementation.
Methods: Using an integrated health system and enterprise data warehouse, we conducted a multi-center mixed academic-community cohort study. FC orders between 2012-2024 were identified, and attributes related to patient demographics, ordering providers, and lab orders including completion rates and time to completion were characterized. Temporal analyses were performed to characterize changes in ordering and completion patterns in relation to key events (COVID pandemic, AGA biomarker guideline release, change in health system lab testing with 48-hour FC turnaround time). Univariate and multivariate logistic regression analyses were performed to identify predictors of FC completion.
Results: We identified 28,424 patients (n=7,353 IBD) with 77,314 FC orders (n=33,720 IBD FC orders). Overall FC completion rates were low (37%) and they were lowest among IBD patients (34%). Temporal analyses identified a significant increase in FC ordering during the COVID pandemic, with no change in completion rates. The release of the AGA biomarker guidelines and change in internal lab turnaround time for test results had no impact on FC ordering or completion rates. Multivariate analyses identified that male patients (adjusted OR (aOR) 1.05, 95% Confidence Interval (CI) 1.01-1.10, P=.027), patients over the age of 65 (aOR 1.15, 95% CI 1.06-1.25, P< .001), those with private insurance (aOR 1.07, 95% CI 1.00-1.15, P=.040), non-white patients (aOR 1.13, 95% CI 1.06-1.20, P< .001), and those cared for in a community setting (aOR 2.07, 95% CI 1.97-2.17, P< .001), were significantly more likely to complete FC testing.
Discussion: In this large cohort study that spanned academic and community locations, we observed low completion rates for FC. Despite a significant increase in ordering during the COVID pandemic, completion rates did not improve. We identified several factors associated with increased FC completion and these data suggest that community-based integration of biomarker-based monitoring is feasible but requires effective strategies to optimize successful implementation.
Disclosures:
Matthew Stanton indicated no relevant financial relationships.
Saihej Basra indicated no relevant financial relationships.
Cenfu Wei indicated no relevant financial relationships.
Stephen B. Hanauer: Abbvie – Consultant. Johnson and Johnson – Consultant. Takeda – Consultant.
Parambir Dulai: AbbVie – Consultant. Abivax – Consultant. Adiso – Consultant. Bristol Meyer Squibb – Consultant. Digbi Health – Royalties. Digbi Health – Stock Options. Geneoscopy – Consultant. GSK – Consultant. Janssen – Consultant. Lilly – Consultant. Pfizer – Consultant, Grant/Research Support. Precidiag – Licensing royalties. Takeda – Consultant, Grant/Research Support.
Matthew B. Stanton, MD1, Saihej P. Basra, 2, Cenfu Wei, MS1, Stephen B. Hanauer, MD, FACG3, Parambir S. Dulai, MD2. P4321 - Fecal Calprotectin Completion Patterns in a Mixed Academic-Community Health System, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
