Tuesday Poster Session
Category: IBD
P4331 - Impact of GLP-1 Agonists on the Severity of Inflammatory Bowel Disease
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- SC
Sarah Coats, MD
Washington University School of Medicine in St. Louis / Barnes-Jewish Hospital
St. Louis, MO
Presenting Author(s)
Sarah Coats, MD1, Scott R. Anderson, MD2, Malek Ayoub, MD2, Parakkal Deepak, MBBS, MS3, Scott McHenry, MD, MSc1
1Washington University School of Medicine in St. Louis / Barnes-Jewish Hospital, St. Louis, MO; 2Washington University School of Medicine in St. Louis, St. Louis, MO; 3Washington University in St. Louis, St. Louis, MO
Introduction: The use of GLP-1 agonists has gained significant popularity for the treatment of type 2 diabetes and obesity, particularly in patients with co-morbid conditions. Beyond their metabolic benefits, GLP-1 agonists are postulated to possess anti-inflammatory properties. However, this area needs more investigation.
Methods: A retrospective chart review was conducted at a tertiary care center on patients diagnosed with inflammatory bowel disease (IBD) who were prescribed any GLP-1 agonist (semaglutide, liraglutide, dulaglutide, exenatide, or tirzepatide) for a minimum duration of 30 days between 2014 and 2024. The primary outcomes evaluated to assess IBD disease severity included the number of IBD-related hospitalizations, clinical disease severity as measured by the Harvey-Bradshaw Index (HBI) for Crohn's Disease (CD) and the Mayo Score/Disease Activity Index (DAI) for Ulcerative Colitis (UC), endoscopic disease activity scores using the Simple Endoscopic Score for Crohn's Disease (SES-CD) and the Mayo Endoscopic Score for Ulcerative Colitis, and the inflammatory marker C-reactive protein (CRP). The Wilcoxon signed-rank test was utilized to compare IBD severity within the year preceding and following the initiation of GLP-1 agonist therapy.
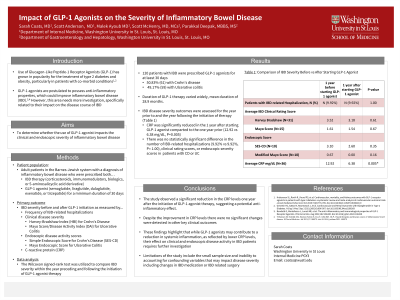
Results: A total of 120 IBD patients (61 CD, 59 UC) who were on GLP-1 agonists for at least 30 days were identified. The mean duration of GLP-1 therapy was 28.9 months. A significant reduction in the average CRP levels was observed one year after starting therapy compared to one year prior (12.92 vs 6.38 mg/dL, P=0.005). However, there was no statistically significant difference in the number of IBD-related hospitalizations. Additionally, no significant changes were observed in clinical rating scores, or endoscopic scores (Table 1).
Discussion: The study observed a significant reduction in the CRP levels one year after the initiation of GLP-1 agonist therapy, suggesting a potential anti-inflammatory effect. Despite the improvement in CRP levels, no significant changes were detected in other key clinical outcomes. These findings highlight that while GLP-1 agonists may contribute to a reduction in systemic inflammation, as reflected by lower CRP levels, their effect on clinical and endoscopic disease activity in IBD patients requires further investigation.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Sarah Coats, MD1, Scott R. Anderson, MD2, Malek Ayoub, MD2, Parakkal Deepak, MBBS, MS3, Scott McHenry, MD, MSc1. P4331 - Impact of GLP-1 Agonists on the Severity of Inflammatory Bowel Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Washington University School of Medicine in St. Louis / Barnes-Jewish Hospital, St. Louis, MO; 2Washington University School of Medicine in St. Louis, St. Louis, MO; 3Washington University in St. Louis, St. Louis, MO
Introduction: The use of GLP-1 agonists has gained significant popularity for the treatment of type 2 diabetes and obesity, particularly in patients with co-morbid conditions. Beyond their metabolic benefits, GLP-1 agonists are postulated to possess anti-inflammatory properties. However, this area needs more investigation.
Methods: A retrospective chart review was conducted at a tertiary care center on patients diagnosed with inflammatory bowel disease (IBD) who were prescribed any GLP-1 agonist (semaglutide, liraglutide, dulaglutide, exenatide, or tirzepatide) for a minimum duration of 30 days between 2014 and 2024. The primary outcomes evaluated to assess IBD disease severity included the number of IBD-related hospitalizations, clinical disease severity as measured by the Harvey-Bradshaw Index (HBI) for Crohn's Disease (CD) and the Mayo Score/Disease Activity Index (DAI) for Ulcerative Colitis (UC), endoscopic disease activity scores using the Simple Endoscopic Score for Crohn's Disease (SES-CD) and the Mayo Endoscopic Score for Ulcerative Colitis, and the inflammatory marker C-reactive protein (CRP). The Wilcoxon signed-rank test was utilized to compare IBD severity within the year preceding and following the initiation of GLP-1 agonist therapy.
Results: A total of 120 IBD patients (61 CD, 59 UC) who were on GLP-1 agonists for at least 30 days were identified. The mean duration of GLP-1 therapy was 28.9 months. A significant reduction in the average CRP levels was observed one year after starting therapy compared to one year prior (12.92 vs 6.38 mg/dL, P=0.005). However, there was no statistically significant difference in the number of IBD-related hospitalizations. Additionally, no significant changes were observed in clinical rating scores, or endoscopic scores (Table 1).
Discussion: The study observed a significant reduction in the CRP levels one year after the initiation of GLP-1 agonist therapy, suggesting a potential anti-inflammatory effect. Despite the improvement in CRP levels, no significant changes were detected in other key clinical outcomes. These findings highlight that while GLP-1 agonists may contribute to a reduction in systemic inflammation, as reflected by lower CRP levels, their effect on clinical and endoscopic disease activity in IBD patients requires further investigation.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Sarah Coats indicated no relevant financial relationships.
Scott Anderson indicated no relevant financial relationships.
Malek Ayoub indicated no relevant financial relationships.
Parakkal Deepak: AbbVie – Consultant, Grant/Research Support. Alimentiv – Grant/Research Support. Arena Pharmaceuticals – Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb-Celgene – Advisory Committee/Board Member, Grant/Research Support. CorEvitas LLC – Consultant. Janssen – Grant/Research Support. Pfizer – Grant/Research Support. Prometheus Biosciences – Grant/Research Support. Roche/Genentech – Advisory Committee/Board Member. Scipher Medicine – Grant/Research Support. Takeda – Grant/Research Support.
Scott McHenry indicated no relevant financial relationships.
Sarah Coats, MD1, Scott R. Anderson, MD2, Malek Ayoub, MD2, Parakkal Deepak, MBBS, MS3, Scott McHenry, MD, MSc1. P4331 - Impact of GLP-1 Agonists on the Severity of Inflammatory Bowel Disease, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
