Tuesday Poster Session
Category: IBD
P4357 - Early Symptomatic Improvement With Mirikizumab Induction Therapy in Patients With Moderately to Severely Active Crohn’s Disease: Results From the Phase 3 VIVID-1 Study
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Bruce E. Sands, MD, FACG
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Bruce E.. Sands, MD, FACG1, Minhu Chen, 2, Silvio Danese, MD, PhD3, Monika Fischer, MD, MS4, Tadakazu Hisamatsu, MD, PhD5, Sami Hoque, MD6, Laurent Peyrin-Biroulet, MD, PhD7, Frank Seibold, 8, Frederick Durand, 9, Zhantao Lin, PhD9, Michelle Lopes, 9, Nathan Morris, 9, Emily Hon, 9, Geert R. D'Haens, MD, PhD10
1Icahn School of Medicine at Mount Sinai, New York, NY; 2First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China; 3Humanitas Clinical and Research Center - IRCCS, Rozzano and Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 4Indiana University, Indianapolis, IN; 5Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 6Barts Health NHS Trust, London, England, United Kingdom; 7INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 8Crohn-Colitis Zentrum, Hochhaus Lindenhofspital, Canton, Bern, Switzerland; 9Eli Lilly and Company, Indianapolis, IN; 10Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands
Introduction: Mirikizumab (miri), an anti-IL-23p19 antibody, demonstrated robust efficacy in improving clinical, endoscopic, and inflammatory biomarker endpoints, and an acceptable safety profile for patients (pts) with Crohn’s disease (CD) in the Phase 3 VIVID-1 study. We investigated the onset of symptomatic improvement and safety of miri as induction therapy through Week 12 (W12) for pts with CD.
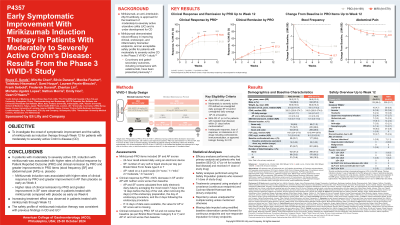
Methods: In VIVID-1, 1065 pts had moderately to severely active CD (unweighted daily average stool frequency (SF) ≥4 [loose and watery stools defined as Bristol Stool Scale Category 6 or 7] AND/OR unweighted daily average abdominal pain (AP) ≥2 at baseline) and prior failure to biologic and/or conventional therapy. Pts were randomized in a 6:3:2 ratio to receive miri (single 900mg intravenous (IV) dose at W0, W4, and W8 followed by a subcutaneous (SC) dose of 300mg at W12 and then every 4 weeks), ustekinumab (uste) (6mg/kg IV at W0 and 90mg SC dose at W8 and then every 8 weeks), or placebo (PBO). This analysis compared clinical response by Patient Reported Outcome (PRO) (30% decrease in SF and/or AP with neither score worse than W0), clinical remission by PRO (SF≤3 and not worse than W0 and AP ≤1 and no worse than W0), change from baseline (CFB) in SF, and in AP up to W12 in miri/PBO randomized patients.
Results: Baseline characteristics were balanced across the miri (N=579) and PBO (n=199) treatment groups. A greater proportion of the pts on miri achieved clinical response by PRO at W4, W6, W8, and W12, and clinical remission by PRO at W6, W8, and W12 vs PBO. Miri resulted in significant improvements in SF at W6, W8, and W12, and in AP at W4, W6, W8, and W12 vs PBO (Table). The overall safety was consistent with the known safety profile of miri. Most common treatment-emergent adverse events (TEAEs) during induction period in miri-treated pts: COVID-19, anemia, and headache. There were lower frequencies of SAEs (miri: 5.9%, PBO: 9.0%) and discontinuations due to adverse events (miri: 2.4%, PBO:4.7%) in miri-treated pts vs PBO. There were no malignancies, no major adverse cardiovascular events (MACEs), no Hy’s law cases or deaths in the miri arm up to W12. One death was reported in the induction period in the PBO group.
Discussion: In pts with moderately to severely active CD, miri induction was associated with higher rates of clinical response, clinical remission and greater improvement in AP as early as W4, SF as early as W6 vs PBO. Increasing treatment effect was observed through W12.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Bruce E.. Sands, MD, FACG1, Minhu Chen, 2, Silvio Danese, MD, PhD3, Monika Fischer, MD, MS4, Tadakazu Hisamatsu, MD, PhD5, Sami Hoque, MD6, Laurent Peyrin-Biroulet, MD, PhD7, Frank Seibold, 8, Frederick Durand, 9, Zhantao Lin, PhD9, Michelle Lopes, 9, Nathan Morris, 9, Emily Hon, 9, Geert R. D'Haens, MD, PhD10. P4357 - Early Symptomatic Improvement With Mirikizumab Induction Therapy in Patients With Moderately to Severely Active Crohn’s Disease: Results From the Phase 3 VIVID-1 Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China; 3Humanitas Clinical and Research Center - IRCCS, Rozzano and Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 4Indiana University, Indianapolis, IN; 5Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 6Barts Health NHS Trust, London, England, United Kingdom; 7INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 8Crohn-Colitis Zentrum, Hochhaus Lindenhofspital, Canton, Bern, Switzerland; 9Eli Lilly and Company, Indianapolis, IN; 10Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands
Introduction: Mirikizumab (miri), an anti-IL-23p19 antibody, demonstrated robust efficacy in improving clinical, endoscopic, and inflammatory biomarker endpoints, and an acceptable safety profile for patients (pts) with Crohn’s disease (CD) in the Phase 3 VIVID-1 study. We investigated the onset of symptomatic improvement and safety of miri as induction therapy through Week 12 (W12) for pts with CD.
Methods: In VIVID-1, 1065 pts had moderately to severely active CD (unweighted daily average stool frequency (SF) ≥4 [loose and watery stools defined as Bristol Stool Scale Category 6 or 7] AND/OR unweighted daily average abdominal pain (AP) ≥2 at baseline) and prior failure to biologic and/or conventional therapy. Pts were randomized in a 6:3:2 ratio to receive miri (single 900mg intravenous (IV) dose at W0, W4, and W8 followed by a subcutaneous (SC) dose of 300mg at W12 and then every 4 weeks), ustekinumab (uste) (6mg/kg IV at W0 and 90mg SC dose at W8 and then every 8 weeks), or placebo (PBO). This analysis compared clinical response by Patient Reported Outcome (PRO) (30% decrease in SF and/or AP with neither score worse than W0), clinical remission by PRO (SF≤3 and not worse than W0 and AP ≤1 and no worse than W0), change from baseline (CFB) in SF, and in AP up to W12 in miri/PBO randomized patients.
Results: Baseline characteristics were balanced across the miri (N=579) and PBO (n=199) treatment groups. A greater proportion of the pts on miri achieved clinical response by PRO at W4, W6, W8, and W12, and clinical remission by PRO at W6, W8, and W12 vs PBO. Miri resulted in significant improvements in SF at W6, W8, and W12, and in AP at W4, W6, W8, and W12 vs PBO (Table). The overall safety was consistent with the known safety profile of miri. Most common treatment-emergent adverse events (TEAEs) during induction period in miri-treated pts: COVID-19, anemia, and headache. There were lower frequencies of SAEs (miri: 5.9%, PBO: 9.0%) and discontinuations due to adverse events (miri: 2.4%, PBO:4.7%) in miri-treated pts vs PBO. There were no malignancies, no major adverse cardiovascular events (MACEs), no Hy’s law cases or deaths in the miri arm up to W12. One death was reported in the induction period in the PBO group.
Discussion: In pts with moderately to severely active CD, miri induction was associated with higher rates of clinical response, clinical remission and greater improvement in AP as early as W4, SF as early as W6 vs PBO. Increasing treatment effect was observed through W12.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Agomab – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Other support, Speakers Bureau. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Enthera – Consultant. Envied Biosciences – Consultant. Equilium – Consultant. Evommune – Consultant. Ferring – Consultant. Fiat – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. Glaxo SmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen – Consultant, Grant/Research Support, Other support, Speakers Bureau. Kaleido – Consultant. Kallyope – Consultant. Lilly – Consultant, other support, Speakers Bureau. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, Other support, Speakers Bureau. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, Other support, Speakers Bureau. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biopharma – Consultant, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Minhu Chen: AbbVie – Provided educational activities. Boehringer Ingelheim – Advisor or Review Panel Member. China Medical System – Provided educational activities. IPSEN – Provided educational activities. Janssen – Advisor or Review Panel Member, Grant/Research Support, Provided educational activities. Takeda – Grant/Research Support, Provided educational activities.
Silvio Danese: AbbVie – Consultant, Speakers Bureau. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Speakers Bureau. Applied Molecular Transport – Consultant. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Celltrion Healthcare – Consultant. Dr Falk Pharma – Consultant. Eli Lilly and Company – Consultant. Enthera – Consultant. Ferring – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. Hospira – Consultant. Inotrem – Consultant. Janssen – Consultant, Speakers Bureau. Johnson & Johnson – Consultant. Morphic – Consultant. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Speakers Bureau. Roche – Consultant. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Speakers Bureau. Teladoc Health – Consultant. TiGenix – Consultant. UCB Inc. – Consultant. Vial – Consultant. Vifor – Consultant.
Monika Fischer: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Eli Lilly and Company – Consultant. Ferring Pharmaceuticals – Consultant. Janssen – Consultant. Pfizer – Consultant. Rebiotix – Consultant. Scioto Biosciences – Consultant. Seres Therapeutics – Consultant.
Tadakazu Hisamatsu: AbbVie – Grant/Research Support, lecture fees. Bristol Myers Squibb – Consultant. Daiichi-Sankyo – Grant/Research Support. EA Pharma – Consultant, Grant/Research Support, lecture fees. Gilead Sciences – Consultant. Janssen – Consultant. JIMRO – Grant/Research Support. Mitsubishi Tanabe Pharma Corporation – Grant/Research Support, lecture fees. Mochida Pharmaceutical – Grant/Research Support. Nippon Kayaku – Grant/Research Support. Pfizer – Grant/Research Support. Takeda Pharmaceutical – Grant/Research Support, lecture fees.
Sami Hoque: Eli Lilly and Company – Advisory Committee/Board Member. Tillots – Advisory Committee/Board Member.
Laurent Peyrin-Biroulet: AbbVie – Grant/Research Support, Personal fees. Allergan – Personal Fees. Alma Bio Therapeutics – Personal Fees. Amgen – Personal Fees. Applied Molecular Transport – Personal Fees. Arena – Personal Fees. Biogen – Personal Fees. Boehringer Ingelheim – Personal Fees. Bristol Myers Squibb – Personal Fees. Celgene – Personal Fees. Celltrion – Personal Fees. CTMA – Stock Options. Enterome – Personal Fees. Enthera – Personal Fees. Ferring – Personal Fees. Fresenius Kabi – Personal Fees. Genentech – Personal Fees. Gilead – Personal Fees. Hikma – Personal Fees. InDex Pharmaceuticals – Personal Fees. Janssen – Personal Fees. Lilly – Personal Fees. MSD – Grant/Research Support, Personal Fees. Mylan – Personal Fees. Nestlé – Personal Fees. Norgine – Personal Fees. Oppilan Pharma – Personal Fees. OSE Immunotherapeutics – Personal Fees. Pfizer Inc – Personal Fees. Pharmacosmos – Fees. Samsung Bioepis – Personal Fees. Sandoz – Personal Fees. Sterna – Personal Fees. Sublimity Therapeutics – Personal Fees. Takeda – Grant/Research Support, Personal Fees. Tillotts – Personal Fees. Vifor – Personal Fees.
Frank Seibold: Abbvie – Consultant. Amgen – Consultant. BMS – Consultant. Dr. Falk – Consultant. Eli Lilly and Company – Consultant. Geilead – Consultant. Janssen – Consultant. Pfizer – Consultant. Takeda – Consultant.
Frederick Durand: Eli Lilly and Company – Employee, Stock Options.
Zhantao Lin: Eli Lilly and Company – Employee, Stock Options.
Michelle Lopes: Eli Lilly and Company – Employee, Stock Options.
Nathan Morris: Eli Lilly and Company – Employee, Stock Options.
Emily Hon: Eli Lilly and Company – Employee, Stock Options.
Geert D'Haens: AbbVie – Advisor or Review Panel Member, Speakers Bureau. Agomab Therapeutics – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. Allergan – Advisor or Review Panel Member. Alphabiomics – Advisor or Review Panel Member. AstraZeneca – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Speakers Bureau. Ferring – Advisor or Review Panel Member. Galapagos – Advisor or Review Panel Member, Speakers Bureau. GlaxoSmithKline – Advisor or Review Panel Member. Immunic – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Seres – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau. Tillotts – Advisor or Review Panel Member, Speakers Bureau. Ventyx – Advisor or Review Panel Member.
Bruce E.. Sands, MD, FACG1, Minhu Chen, 2, Silvio Danese, MD, PhD3, Monika Fischer, MD, MS4, Tadakazu Hisamatsu, MD, PhD5, Sami Hoque, MD6, Laurent Peyrin-Biroulet, MD, PhD7, Frank Seibold, 8, Frederick Durand, 9, Zhantao Lin, PhD9, Michelle Lopes, 9, Nathan Morris, 9, Emily Hon, 9, Geert R. D'Haens, MD, PhD10. P4357 - Early Symptomatic Improvement With Mirikizumab Induction Therapy in Patients With Moderately to Severely Active Crohn’s Disease: Results From the Phase 3 VIVID-1 Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
