Tuesday Poster Session
Category: IBD
P4374 - Understanding Gastroenterologist Preferences at the Time of Treatment Escalation to First-Line Advanced Therapies in Ulcerative Colitis: A Discrete Choice Experiment in Five European Countries
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- PH
Peter Hur, PharmD
Pfizer Inc.
New York, NY
Presenting Author(s)
Stefan Schreiber, MD1, Alissa Walsh, MD2, Peter Hur, PharmD3, Laura Panattoni, PhD4, Brett Hauber, 3, Grace Gahlon, 4, Josh Coulter, 3, Karolina Wosik, MSc, PhD5, Joseph C. Cappelleri, PhD, MPH6, Nadya Prood, MPH4, Xiang Guo, PharmD, MS7, Anthony Buisson, MD, PhD8
1University Hospital, Kiel, Schleswig-Holstein, Germany; 2Translational Gastroenterology Unit, Oxford University Hospital, Oxford, England, United Kingdom; 3Pfizer Inc., New York, NY; 4PRECISIONheor, New York, NY; 5Pfizer Canada, Kirkland, PQ, Canada; 6Pfizer Inc., Groton, CT; 7Pfizer Inc., Collegeville, PA; 8University Hospital Estaing, Clermont Auvergne University, Inserm U1071, M2iSH, USC-INRA 2018, Clermont-Ferrand, Auvergne, France
Introduction: As the number of advanced treatment options for moderately to severely active ulcerative colitis (UC) increases, it is necessary to understand the factors driving gastroenterologist (GE) choice when escalating patients from conventional to advanced therapy.
Methods: We completed a quantitative analysis of GE therapy attribute preferences when choosing to escalate patients to their first advanced UC therapy. We conducted an online cross-sectional survey using a discrete choice experiment (DCE) design. Attribute and level selection was informed by a targeted literature search and formative qualitative research with patients and clinicians. Survey responders were practicing GEs experienced in treating patients with moderately to severely active UC, recruited from France, Germany, Italy, Spain, and the United Kingdom (UK). Preference weights were estimated using a random parameters logit model for varying levels of seven attributes: time to symptom improvement, probability of remission at one year, difference between probability of remission and CS-free remission, five-year risk of malignancy, annual risk of serious infection, annual risk of major adverse cardiovascular events, and mode and frequency of administration. Relative importance (RI) was calculated using the difference in preference weights between the most and least preferred level of each attribute, scaled from 0 to 100%. An additional survey section was included to understand GE treatment and prescribing practices.
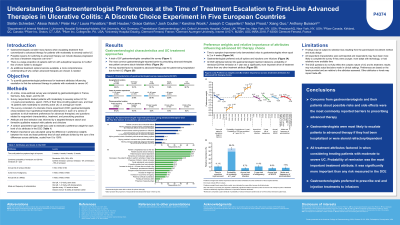
Results: A total of 397 GEs were included (France n = 140; Germany n = 40; Italy n = 40; Spain n = 47; UK n = 130). The most common GE-reported barriers to prescribing advanced therapies were concerns about contraindications and risks/side effects from patients (54.9%) and GEs (39.8%), perceived patient concerns about receiving injections or infusions (35.5%), and concerns about cost or insufficient reimbursement (32.2%). All DCE attributes factored into GE treatment decisions (see Table for RI and preference weights). The three most impactful attributes were probability of remission at one year (RI 48.4%), followed by 5-year risk of malignancy (RI 11.4%) and time to symptom improvement (RI 11.1%; Table).
Discussion: All attributes factored into the trade-offs GEs consider when escalating patients with moderately to severely active UC to their first advanced therapy. Whereas risk of side effects was the most stated GE barrier to prescribing advanced therapy, probability of remission outweighed all other DCE attributes.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Stefan Schreiber, MD1, Alissa Walsh, MD2, Peter Hur, PharmD3, Laura Panattoni, PhD4, Brett Hauber, 3, Grace Gahlon, 4, Josh Coulter, 3, Karolina Wosik, MSc, PhD5, Joseph C. Cappelleri, PhD, MPH6, Nadya Prood, MPH4, Xiang Guo, PharmD, MS7, Anthony Buisson, MD, PhD8. P4374 - Understanding Gastroenterologist Preferences at the Time of Treatment Escalation to First-Line Advanced Therapies in Ulcerative Colitis: A Discrete Choice Experiment in Five European Countries, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University Hospital, Kiel, Schleswig-Holstein, Germany; 2Translational Gastroenterology Unit, Oxford University Hospital, Oxford, England, United Kingdom; 3Pfizer Inc., New York, NY; 4PRECISIONheor, New York, NY; 5Pfizer Canada, Kirkland, PQ, Canada; 6Pfizer Inc., Groton, CT; 7Pfizer Inc., Collegeville, PA; 8University Hospital Estaing, Clermont Auvergne University, Inserm U1071, M2iSH, USC-INRA 2018, Clermont-Ferrand, Auvergne, France
Introduction: As the number of advanced treatment options for moderately to severely active ulcerative colitis (UC) increases, it is necessary to understand the factors driving gastroenterologist (GE) choice when escalating patients from conventional to advanced therapy.
Methods: We completed a quantitative analysis of GE therapy attribute preferences when choosing to escalate patients to their first advanced UC therapy. We conducted an online cross-sectional survey using a discrete choice experiment (DCE) design. Attribute and level selection was informed by a targeted literature search and formative qualitative research with patients and clinicians. Survey responders were practicing GEs experienced in treating patients with moderately to severely active UC, recruited from France, Germany, Italy, Spain, and the United Kingdom (UK). Preference weights were estimated using a random parameters logit model for varying levels of seven attributes: time to symptom improvement, probability of remission at one year, difference between probability of remission and CS-free remission, five-year risk of malignancy, annual risk of serious infection, annual risk of major adverse cardiovascular events, and mode and frequency of administration. Relative importance (RI) was calculated using the difference in preference weights between the most and least preferred level of each attribute, scaled from 0 to 100%. An additional survey section was included to understand GE treatment and prescribing practices.
Results: A total of 397 GEs were included (France n = 140; Germany n = 40; Italy n = 40; Spain n = 47; UK n = 130). The most common GE-reported barriers to prescribing advanced therapies were concerns about contraindications and risks/side effects from patients (54.9%) and GEs (39.8%), perceived patient concerns about receiving injections or infusions (35.5%), and concerns about cost or insufficient reimbursement (32.2%). All DCE attributes factored into GE treatment decisions (see Table for RI and preference weights). The three most impactful attributes were probability of remission at one year (RI 48.4%), followed by 5-year risk of malignancy (RI 11.4%) and time to symptom improvement (RI 11.1%; Table).
Discussion: All attributes factored into the trade-offs GEs consider when escalating patients with moderately to severely active UC to their first advanced therapy. Whereas risk of side effects was the most stated GE barrier to prescribing advanced therapy, probability of remission outweighed all other DCE attributes.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Stefan Schreiber: AbbVie – Consultant, Personal fees, Speakers Bureau. Amgen – Personal fees. Arena Pharmaceuticals – Consultant, Personal fees, Speakers Bureau. Biogen – Consultant, Personal fees, Speakers Bureau. Bristol Myers Squibb – Consultant, Personal fees, Speakers Bureau. Celgene – Consultant, Personal fees, Speakers Bureau. Celltrion – Consultant, Personal fees, Speakers Bureau. Eli Lilly and Company – Personal fees. Falk – Consultant, Personal fees, Speakers Bureau. Ferring Pharmaceuticals – Personal fees. Fresenius – Consultant, Personal fees, Speakers Bureau. Galapagos – Personal fees. Gilead – Consultant, Personal fees. Hikma Pharmaceuticals – Advisory Committee/Board Member, Consultant. I-MAB – Consultant, Personal fees. Janssen – Consultant, Personal fees, Speakers Bureau. Morphic – Personal fees. MSD – Consultant, Personal fees, Speakers Bureau. Mylan – Consultant, Personal fees. Novartis – Personal fees. Pfizer Inc – Consultant, Personal fees, Speakers Bureau. Protagonist – Consultant, Personal fees. Provention Bio – Consultant, Personal fees. Roche – Personal fees. Sandoz/Hexal – Personal fees. Shire – Personal fees. Takeda – Consultant, Personal fees, Speakers Bureau. Theravance Biopharma – Consultant, Personal fees. Ventyx – Consultant, Personal fees.
Alissa Walsh: AbbVie – Consultant, Grant/Research Support. Bühlmann Laboratories – Consultant, Grant/Research Support. Eli Lilly – Consultant, Grant/Research Support. Galapagos – Consultant, Grant/Research Support. GlaxoSmithKline – Consultant, Grant/Research Support. Janssen – Consultant, Grant/Research Support. Pfizer Inc – Consultant, Grant/Research Support. Takeda – Consultant, Grant/Research Support. Vifor – Consultant, Grant/Research Support.
Peter Hur: Clene Nanomedicine – Stock Options. Haleon – Stock Options. Isdoria – Stock Options. Liquidia – Stock Options. Longboard Pharmaceuticals – Stock Options. Pfizer Inc – Employee, Stock Options. Proctor Gamble – Stock Options. US 2022/0257594 A1 – Intellectual Property/Patents.
Laura Panattoni: AHRQ – Grant/Research Support. American Cancer Society – Grant/Research Support. Fred Hutchinson Cancer Research Center – Grant/Research Support. Gordon and Betty Moore Foundation – Grant/Research Support. ISPOR Planning Committe – Advisor or Review Panel Member. Microsoft – Grant/Research Support. NCI – Grant/Research Support. NIH – Grant/Research Support. Precision AQ – Consultant, Employee, Stock Options.
Brett Hauber: Pfizer Inc – Employee, Stock Options.
Grace Gahlon: PRECISIONheor – Consultant.
Josh Coulter: Pfizer Inc – Employee, Stock Options.
Karolina Wosik: Pfizer Canada Inc – Employee, Stock Options.
Joseph Cappelleri: Pfizer Inc – Employee, Stock Options.
Nadya Prood: PRECISIONheor – Consultant.
Xiang Guo: Pfizer Inc – Employee, Stock Options.
Anthony Buisson: AbbVie – Consultant, Speakers Bureau. Amgen – Consultant, Speakers Bureau. Arena – Consultant, Speakers Bureau. Biogen – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Mayoly-Spindler – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Norgine – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Speakers Bureau. Roche – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau. Tillotts – Consultant, Speakers Bureau.
Stefan Schreiber, MD1, Alissa Walsh, MD2, Peter Hur, PharmD3, Laura Panattoni, PhD4, Brett Hauber, 3, Grace Gahlon, 4, Josh Coulter, 3, Karolina Wosik, MSc, PhD5, Joseph C. Cappelleri, PhD, MPH6, Nadya Prood, MPH4, Xiang Guo, PharmD, MS7, Anthony Buisson, MD, PhD8. P4374 - Understanding Gastroenterologist Preferences at the Time of Treatment Escalation to First-Line Advanced Therapies in Ulcerative Colitis: A Discrete Choice Experiment in Five European Countries, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
