Tuesday Poster Session
Category: IBD
P4378 - European and U.S. Patient and Health Care Professional Perspectives on the Experience of Fatigue in Crohn’s Disease and Ulcerative Colitis: Communicating Needs and Features of IBD Experiences (CONFIDE) Survey
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Remo Panaccione, MD
University of Calgary
Calgary, AB, Canada
Presenting Author(s)
Remo Panaccione, MD1, Alison Bleakman, 2, Stefan Schreiber, MD3, Simon Travis, 4, Marla C. Dubinsky, MD5, Toshifumi Hibi, MD, PhD6, Theresa Gibble, PhD2, Tommaso Panni, PhD2, Cem Kayhan, MD2, Eoin Flynn, PhD2, Angelo Favia, PhD2, Christian Atkinson, MRes7, David Rubin, 8
1University of Calgary, Calgary, AB, Canada; 2Eli Lilly and Company, Indianapolis, IN; 3University Hospital, Kiel, Schleswig-Holstein, Germany; 4University of Oxford, Oxford, England, United Kingdom; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6Kitasato University, Tokyo, Japan, Shirokane, Minato-ku, Tokyo, Japan; 7Adelphi Real World, Bollington, England, United Kingdom; 8University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL
Introduction: Fatigue is a prevalent and debilitating, yet under-recognized and under-treated, symptom among patients (pts) with Crohn’s disease (CD) or ulcerative colitis (UC). The Communicating Needs and Features of IBD Experiences (CONFIDE) study explored pt and healthcare professional (HCP) perspectives on the experience and impact of CD/UC-related symptoms in Europe (France, Germany, Italy, Spain, and UK), the United States (US), and Japan.
Methods: Online, quantitative, cross-sectional surveys were conducted separately among pts with moderate-to-severe CD/UC and HCPs who make prescribing decisions for pts. Moderate-to-severe CD/UC were defined based on previous treatment, steroid use, and/or hospitalization. Descriptive statistics were used to summarize the European and US data related to fatigue.
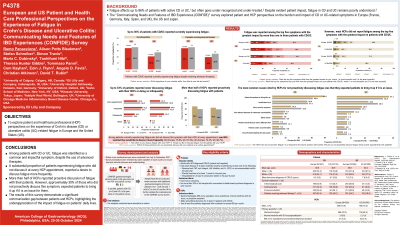
Results: Total 547 European [55.4% male, mean age: 38.0 years (yrs)] and 215 US (54.9% male, 40.9 yrs) pts with CD and 556 European (57.4% male, 38.9 yrs) and 200 US (61.5% male, 40.4 yrs) pts with UC completed the surveys. Over 50% of all pts were receiving advanced therapies. Overall, 34.2% European and 35.8% US pts with CD, and 20.9% European and 27.5% US pts with UC reported currently experiencing fatigue (in the past month). Similar results were observed among those receiving advanced therapies. Over one-third of CD and UC pts ranked fatigue among the top five symptoms with greatest impact on them. Among pts currently experiencing fatigue, most did not discuss it with their HCPs at every appointment, with at least two-thirds of these pts indicating that they would like to discuss it more frequently. Surveys were completed by 503 European (70.8% male) and 200 US (78.0% male) HCPs; 92.5% were gastroenterologists/internal medicine practitioners. A minority of HCPs reported fatigue among the top five symptoms with greatest impact on pts. Over half of HCPs reported proactively discussing fatigue at routine pt appointments. The most common reason for not proactively discussing it was that they expected pts to bring it up if it is an issue (Table).
Discussion: For both pts with CD/UC, fatigue was a common and impactful symptom, despite use of advanced therapies. Most pts experiencing fatigue who did not discuss it at every HCP appointment, wanted to discuss fatigue more frequently. Although >50% HCPs reported proactive discussion of fatigue with their pts, some expected pts to bring it up. This indicates a communication gap and highlights the underappreciation of the impact of fatigue in CD/UC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Remo Panaccione, MD1, Alison Bleakman, 2, Stefan Schreiber, MD3, Simon Travis, 4, Marla C. Dubinsky, MD5, Toshifumi Hibi, MD, PhD6, Theresa Gibble, PhD2, Tommaso Panni, PhD2, Cem Kayhan, MD2, Eoin Flynn, PhD2, Angelo Favia, PhD2, Christian Atkinson, MRes7, David Rubin, 8. P4378 - European and U.S. Patient and Health Care Professional Perspectives on the Experience of Fatigue in Crohn’s Disease and Ulcerative Colitis: Communicating Needs and Features of IBD Experiences (CONFIDE) Survey, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Calgary, Calgary, AB, Canada; 2Eli Lilly and Company, Indianapolis, IN; 3University Hospital, Kiel, Schleswig-Holstein, Germany; 4University of Oxford, Oxford, England, United Kingdom; 5Icahn School of Medicine at Mount Sinai, New York, NY; 6Kitasato University, Tokyo, Japan, Shirokane, Minato-ku, Tokyo, Japan; 7Adelphi Real World, Bollington, England, United Kingdom; 8University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL
Introduction: Fatigue is a prevalent and debilitating, yet under-recognized and under-treated, symptom among patients (pts) with Crohn’s disease (CD) or ulcerative colitis (UC). The Communicating Needs and Features of IBD Experiences (CONFIDE) study explored pt and healthcare professional (HCP) perspectives on the experience and impact of CD/UC-related symptoms in Europe (France, Germany, Italy, Spain, and UK), the United States (US), and Japan.
Methods: Online, quantitative, cross-sectional surveys were conducted separately among pts with moderate-to-severe CD/UC and HCPs who make prescribing decisions for pts. Moderate-to-severe CD/UC were defined based on previous treatment, steroid use, and/or hospitalization. Descriptive statistics were used to summarize the European and US data related to fatigue.
Results: Total 547 European [55.4% male, mean age: 38.0 years (yrs)] and 215 US (54.9% male, 40.9 yrs) pts with CD and 556 European (57.4% male, 38.9 yrs) and 200 US (61.5% male, 40.4 yrs) pts with UC completed the surveys. Over 50% of all pts were receiving advanced therapies. Overall, 34.2% European and 35.8% US pts with CD, and 20.9% European and 27.5% US pts with UC reported currently experiencing fatigue (in the past month). Similar results were observed among those receiving advanced therapies. Over one-third of CD and UC pts ranked fatigue among the top five symptoms with greatest impact on them. Among pts currently experiencing fatigue, most did not discuss it with their HCPs at every appointment, with at least two-thirds of these pts indicating that they would like to discuss it more frequently. Surveys were completed by 503 European (70.8% male) and 200 US (78.0% male) HCPs; 92.5% were gastroenterologists/internal medicine practitioners. A minority of HCPs reported fatigue among the top five symptoms with greatest impact on pts. Over half of HCPs reported proactively discussing fatigue at routine pt appointments. The most common reason for not proactively discussing it was that they expected pts to bring it up if it is an issue (Table).
Discussion: For both pts with CD/UC, fatigue was a common and impactful symptom, despite use of advanced therapies. Most pts experiencing fatigue who did not discuss it at every HCP appointment, wanted to discuss fatigue more frequently. Although >50% HCPs reported proactive discussion of fatigue with their pts, some expected pts to bring it up. This indicates a communication gap and highlights the underappreciation of the impact of fatigue in CD/UC.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Remo Panaccione: Élan – Consultant. Abbivax – Consultant. Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaking Fees. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker Fees. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker Fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker Fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker Fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Ferring – Advisory Committee/Board Member, Consultant, Speaker Fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker Fees. Galapagos – Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant, Speaker Fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Bio – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speaker Fees. Lilly – Advisory Committee/Board Member, Consultant, Speaker Fees. Merck – Advisory Committee/Board Member, Consultant, Speaker Fees. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker Fees. Pandion Pharma – Advisory Committee/Board Member, Consultant. Pendopharm – Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Speaker Fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker Fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker Fees. Satisfai Health – Consultant. Shire – Advisory Committee/Board Member, Consultant, Speaker Fees. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speaker Fees. Theravance – Consultant. Trellus – Consultant. UCB – Consultant. Ventyx – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Alison Bleakman: Eli Lilly and Company – Employee, Stock Options.
Stefan Schreiber: AbbVie – Consultant, Personal fees, Speakers Bureau. Amgen – Personal fees. Arena Pharmaceuticals – Consultant, Personal fees, Speakers Bureau. Biogen – Consultant, Personal fees, Speakers Bureau. Bristol Myers Squibb – Consultant, Personal fees, Speakers Bureau. Celgene – Consultant, Personal fees, Speakers Bureau. Celltrion – Consultant, Personal fees, Speakers Bureau. Eli Lilly and Company – Personal fees. Falk – Consultant, Personal fees, Speakers Bureau. Ferring Pharmaceuticals – Personal fees. Fresenius – Consultant, Personal fees, Speakers Bureau. Galapagos – Personal fees. Gilead – Consultant, Personal fees. Hikma Pharmaceuticals – Advisory Committee/Board Member, Consultant. I-MAB – Consultant, Personal fees. Janssen – Consultant, Personal fees, Speakers Bureau. Morphic – Personal fees. MSD – Consultant, Personal fees, Speakers Bureau. Mylan – Consultant, Personal fees. Novartis – Personal fees. Pfizer Inc – Consultant, Personal fees, Speakers Bureau. Protagonist – Consultant, Personal fees. Provention Bio – Consultant, Personal fees. Roche – Personal fees. Sandoz/Hexal – Personal fees. Shire – Personal fees. Takeda – Consultant, Personal fees, Speakers Bureau. Theravance Biopharma – Consultant, Personal fees. Ventyx – Consultant, Personal fees.
Simon Travis: AbbVie – Grant/Research Support. Buhlmann – Grant/Research Support. ECCO – Grant/Research Support. Eli Lilly and Company – Grant/Research Support. Ferring Pharmaceuticals – Grant/Research Support. International Organization for the Study of IBD – Grant/Research Support. Janssen – Grant/Research Support. MSD – Grant/Research Support. Normal Collision Foundation – Grant/Research Support. Pfizer – Grant/Research Support. Procter & Gamble – Grant/Research Support. Schering-Plough – Grant/Research Support. Takeda – Grant/Research Support. UCB – Grant/Research Support. Vifor Pharma – Grant/Research Support. Warner Chilcott – Grant/Research Support.
Marla Dubinsky: AbbVie – Consultant. Abivax – Consultant. Arena – Consultant. AstraZeneca – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Genentech – Consultant. Gilead – Consultant. Janssen – Consultant. Pfizer Inc – Consultant. Prometheus Labs – Consultant. Takeda – Consultant.
Toshifumi Hibi: AbbVie GK – Grant/Research Support. ActivAid – Grant/Research Support. Alfresa Pharma Corporation – Grant/Research Support, study group sponsorship. Bristol-Myers Squibb – Grant/Research Support. Celltrion – Advisory/consultancy fees. EA Pharma – Lecture fees. Eli Lilly and Company – Grant/Research Support, Advisory/consultancy fees. Ferring Pharmaceuticals – Grant/Research Support. Gilead Sciences – Grant/Research Support. Janssen – Grant/Research Support. JIMRO – study group sponsorship and lecture fees. JMDC Inc. – Grant/Research Support. Kyorin – study group sponsorship and lecture fees. Mitsubishi-Tanabe Pharma Corporation – Advisory/consultancy fees and lecture fees. MIYARISAN Pharmaceutical – study group sponsorship. Mochida Pharmaceutical – Grant/Research Support, study group sponsorship and lecture fees. Nippon Kayaku Co. – Grant/Research Support. Pfizer – Grant/Research Support, Lecture fees. Takeda Pharmaceutical – Grant/Research Support, Advisory/consultancy fees and lecture fees. Zeria Pharmaceutical – study group sponsorship and lecture fees.
Theresa Gibble: Eli Lilly and Company – Employee, Stock Options.
Tommaso Panni: Eli Lilly and Company – Employee. Eli Lilly and Company – Employee, Stock Options. Eli Lilly and Company – Stock Options.
Cem Kayhan: Eli Lilly and Company – Employee, Stock Options.
Eoin Flynn: Eli Lilly and Company – Employee, Stock Options.
Angelo Favia: Eli Lilly and Company – Employee, Stock Options.
Christian Atkinson: Adelphi Real World – Employee. Eli Lilly and Company (in connection with this study) – Consultant.
David Rubin: AbbVie – Consultant. Allergan – Consultant. Altrubio – Stock Options. Altrubio Inc. – Consultant. American College of Gastroenterology – Consultant. Arena Pharmaceuticals – Consultant. Athos Therapeutics – Consultant. Bellatrix Pharmaceuticals – Consultant. Boehringer Ingelheim International GmbH – Consultant. Bristol Myers Squibb – Consultant. Celgene Corp/Syneos – Consultant. Cornerstones Health – Consultant. Datos Health – Stock Options. Eli Lilly and Company – Consultant. GalenPharma/Atlantica – Consultant. Genentech/Roche – Consultant. Gilead Sciences – Consultant. GODURN – Consultant. Inc. (non-profit) – Consultant. InDex Pharmaceuticals – Consultant. Ironwood Pharmaceuticals – Consultant. Iterative Scopes – Consultant. Janssen – Consultant. LLC – Consultant. Materia Prima – Consultant. Pfizer – Consultant. Prometheus Biosciences – Consultant. Reistone Biopharma – Consultant. Takeda – Consultant, Grant/Research Support. Techlab Inc – Consultant.
Remo Panaccione, MD1, Alison Bleakman, 2, Stefan Schreiber, MD3, Simon Travis, 4, Marla C. Dubinsky, MD5, Toshifumi Hibi, MD, PhD6, Theresa Gibble, PhD2, Tommaso Panni, PhD2, Cem Kayhan, MD2, Eoin Flynn, PhD2, Angelo Favia, PhD2, Christian Atkinson, MRes7, David Rubin, 8. P4378 - European and U.S. Patient and Health Care Professional Perspectives on the Experience of Fatigue in Crohn’s Disease and Ulcerative Colitis: Communicating Needs and Features of IBD Experiences (CONFIDE) Survey, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
