Tuesday Poster Session
Category: IBD
P4392 - Multiple Gastrointestinal Immune Related Adverse Events From Immune Checkpoint Inhibitor Therapy
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Trevor Barlowe, MD
University of North Carolina at Chapel Hill School of Medicine
Chapel Hill, NC
Presenting Author(s)
Trevor Barlowe, MD1, Shruti Saxena-Beem, BS1, Rumey Ishizawar, MD, PhD1, Hans Herfarth, MD, PhD1, Andrew Moon, MD, MPH2
1University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC; 2University of North Carolina, Chapel Hill, NC
Introduction: Immune checkpoint inhibitors (ICI) are cancer therapies that activate the host immune response by blocking immune suppressive signaling pathways. Inflammatory side effects, termed immune related adverse events (irAEs), are a serious side effect of ICIs that lead to disruption of therapy. Much of the existing literature and clinical trial data categorize irAEs as single-organ events. However, there is emerging evidence that irAEs can impact multiple organs at once, termed multisystem irAEs, which remain poorly characterized. We therefore aimed to evaluate patients who received ICIs and experienced multiple gastrointestinal irAEs.
Methods: A 2843 patient cohort was identified consisting of all patients with cancer treated with ICIs at a large tertiary cancer center between 2014 and 2019. EMERSE (Electronic Medical Record Search Engine), an information retrieval system, was used to search free text (unstructured) clinical notes in the electronic medical record to identify patients with gastrointestinal irAEs. Chart review was then performed to abstract clinical data.
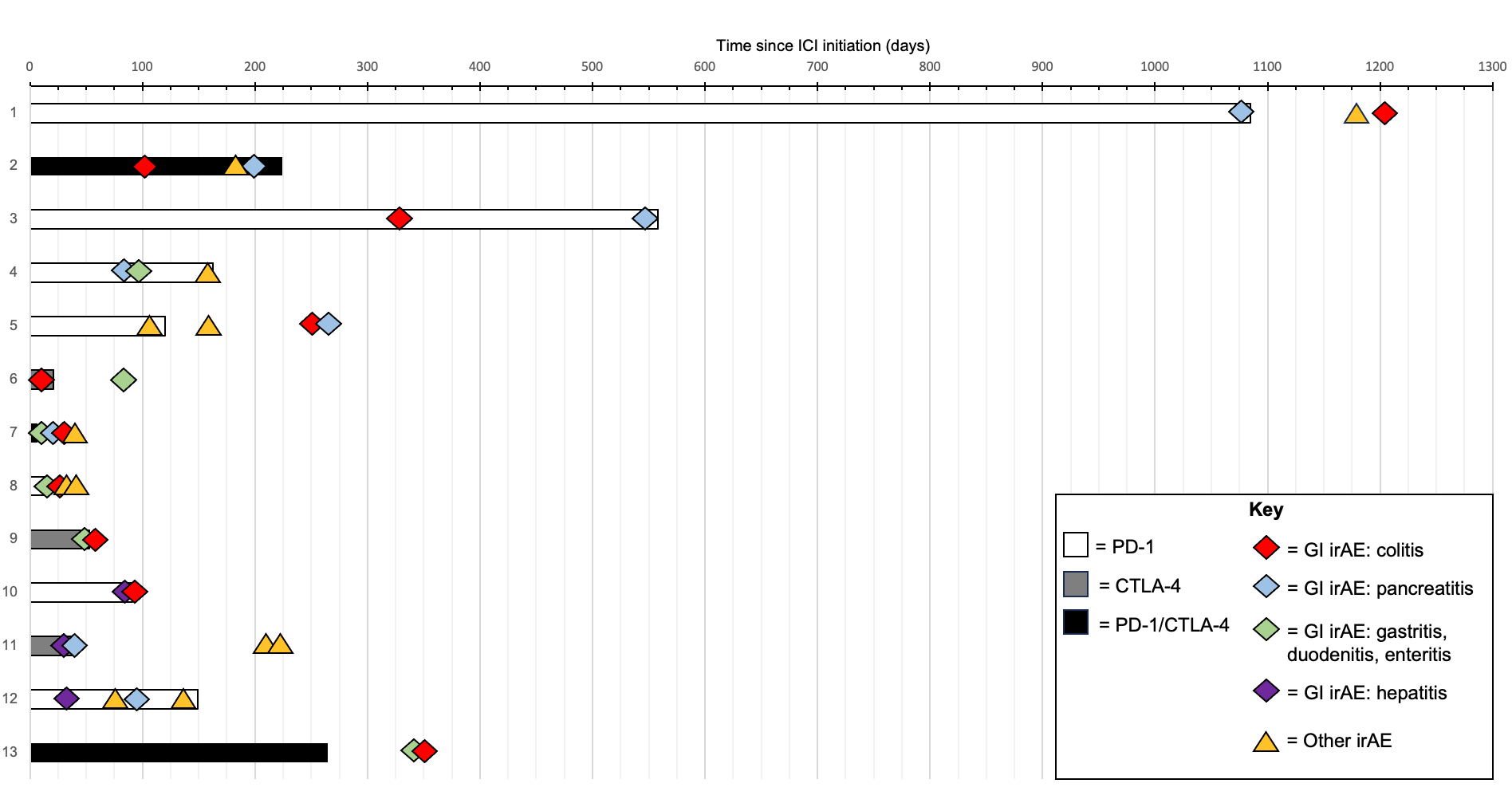
Results: Of the 2843 patients with cancer receiving ICIs, we identified 13 patients who developed multiple gastrointestinal irAEs (0.46%). Clinical characteristics of the identified patients are summarized in Table 1. ICI regimen included anti PD1, anti CTLA-4, or combination anti PD1/anti CTLA-4 for a varying number of cycles (4.0 ± 10.3). 3/13 patients had progression of malignancy while being treated with ICI. The most common single gastrointestinal irAE was colitis (85%), followed by pancreatitis (62%). The most common multisystem gastrointestinal irAE was colitis + pancreatitis (45%), followed by colitis + enteritis (39%). 6/11 patients with colitis needed treatment with biologics due to failure of steroids to control symptoms. 1/4 patients with hepatitis required mycophenolate. The timing of development of irAEs is shown in Figure 1. There was no consistent temporal relationship between ICI administration, gastrointestinal irAEs, or irAEs in other organ systems.
Discussion: This study evaluated a cohort of patients who received ICI and developed multisystem gastrointestinal irAEs. As use of ICIs increase, identification and management of irAE, even rare events, will be important for research and clinical efforts. Future studies should report multisystem irAEs and practitioners who care for patients with irAE should diligently evaluate for systemic toxicities.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Trevor Barlowe, MD1, Shruti Saxena-Beem, BS1, Rumey Ishizawar, MD, PhD1, Hans Herfarth, MD, PhD1, Andrew Moon, MD, MPH2. P4392 - Multiple Gastrointestinal Immune Related Adverse Events From Immune Checkpoint Inhibitor Therapy, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC; 2University of North Carolina, Chapel Hill, NC
Introduction: Immune checkpoint inhibitors (ICI) are cancer therapies that activate the host immune response by blocking immune suppressive signaling pathways. Inflammatory side effects, termed immune related adverse events (irAEs), are a serious side effect of ICIs that lead to disruption of therapy. Much of the existing literature and clinical trial data categorize irAEs as single-organ events. However, there is emerging evidence that irAEs can impact multiple organs at once, termed multisystem irAEs, which remain poorly characterized. We therefore aimed to evaluate patients who received ICIs and experienced multiple gastrointestinal irAEs.
Methods: A 2843 patient cohort was identified consisting of all patients with cancer treated with ICIs at a large tertiary cancer center between 2014 and 2019. EMERSE (Electronic Medical Record Search Engine), an information retrieval system, was used to search free text (unstructured) clinical notes in the electronic medical record to identify patients with gastrointestinal irAEs. Chart review was then performed to abstract clinical data.
Results: Of the 2843 patients with cancer receiving ICIs, we identified 13 patients who developed multiple gastrointestinal irAEs (0.46%). Clinical characteristics of the identified patients are summarized in Table 1. ICI regimen included anti PD1, anti CTLA-4, or combination anti PD1/anti CTLA-4 for a varying number of cycles (4.0 ± 10.3). 3/13 patients had progression of malignancy while being treated with ICI. The most common single gastrointestinal irAE was colitis (85%), followed by pancreatitis (62%). The most common multisystem gastrointestinal irAE was colitis + pancreatitis (45%), followed by colitis + enteritis (39%). 6/11 patients with colitis needed treatment with biologics due to failure of steroids to control symptoms. 1/4 patients with hepatitis required mycophenolate. The timing of development of irAEs is shown in Figure 1. There was no consistent temporal relationship between ICI administration, gastrointestinal irAEs, or irAEs in other organ systems.
Discussion: This study evaluated a cohort of patients who received ICI and developed multisystem gastrointestinal irAEs. As use of ICIs increase, identification and management of irAE, even rare events, will be important for research and clinical efforts. Future studies should report multisystem irAEs and practitioners who care for patients with irAE should diligently evaluate for systemic toxicities.

Figure: Figure 1: Swimmer plot illustrating patients treated with ICI who developed multiple gastrointestinal irAEs
ICI = immune checkpoint inhibitor, GI irAE = gastrointestinal system specific immune related adverse event, Other irAE = immune related adverse event occurring in any other organ system aside from GI system.
ICI = immune checkpoint inhibitor, GI irAE = gastrointestinal system specific immune related adverse event, Other irAE = immune related adverse event occurring in any other organ system aside from GI system.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Trevor Barlowe indicated no relevant financial relationships.
Shruti Saxena-Beem indicated no relevant financial relationships.
Rumey Ishizawar indicated no relevant financial relationships.
Hans Herfarth indicated no relevant financial relationships.
Andrew Moon: DCN Diagnostics – Grant/Research Support. Intercept – Advisory Committee/Board Member. TARGET RWE – Consultant.
Trevor Barlowe, MD1, Shruti Saxena-Beem, BS1, Rumey Ishizawar, MD, PhD1, Hans Herfarth, MD, PhD1, Andrew Moon, MD, MPH2. P4392 - Multiple Gastrointestinal Immune Related Adverse Events From Immune Checkpoint Inhibitor Therapy, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
