Tuesday Poster Session
Category: Interventional Endoscopy
P4482 - Safety and Feasibility of Endoscopic Ultrasonography Guided Liver Biopsy (EUS-LB) in a Free-Standing Ambulatory Surgery Center (ASC)
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Harshaman Kaur, MD
Mountain Vista Medical Center
Phoenix, AZ
Presenting Author(s)
Mankanwal S. Sachdev, MD1, Harshaman Kaur, MD2, Amninder Singh, MD3, Ananya Das, MD4
1AZCDH, Paradise Valley, AZ; 2Mountain Vista Medical Center, Phoenix, AZ; 3The Wright Center for Graduate Medical Education, Scranton, PA; 4Arizona Centers for Digestive Health, Paradise Valley, AZ
Introduction: EUS has been historically limited to hospital-based facilities due to cost, and resources. ASCs offer convenient, safe, and economical options for the performance of standard endoscopy and are attractive to both patients and healthcare payers because of the provision of high-quality care at a significantly lower cost when compared to hospital-based outpatient facilities (Frakes 2006). In a previous study, we showed that EUS procedures could be safely and efficiently performed in an ASC. As EUS techniques have advanced, the range of procedures and interventions has expanded. EUS-LB has increasingly become a common procedure for endosonographers. In this study, we aimed to show that EUS-LB can be done safely and effectively in an ASC.
Methods: We started to perform EUS-LB in our ASC in 2016, and initially these procedures were done sporadically. Since 2022, EUS-LBs have been done more frequently, and this is a retrospective audit of these cases. All patients, ASA 3 or less, requiring EUS were routinely performed in a free-standing ASC by two experienced endosonographers, in a dedicated EUS room equipped with echoendoscopes. Monthly audits were performed to evaluate adverse events by tracking the average time in the facility (from check-in to check-out) Data was collected from the electronic health record as a part of a quality improvement project.
Results: 86 patients underwent EUS-LB, median age was 58 years (IQR 27), 35% were men, 34% white, and median BMI 30.4 (IQR 10.38). Common comorbidities included hypertension (41.9%) hyperlipidemia (33.7%). 51.2% of patients were obese. The primary indications for biopsy were elevated liver enzymes (55.8%) and abnormal non-invasive liver imaging (14%).
Biopsies were mostly performed on the left lobe (98.8%), using fine-needle biopsy (FNB) exclusively. Needle sizes employed were predominantly 19 G (82.6%), followed by 22 G (12.8%) and 25 G (1.2%). Adverse events were minimal, with abdominal pain reported in 27.9% of cases. There were no occurrences of pneumothorax, hemorrhage, hypotension, tumor seeding, or peritonitis. No patients were hospitalized for the adverse events.
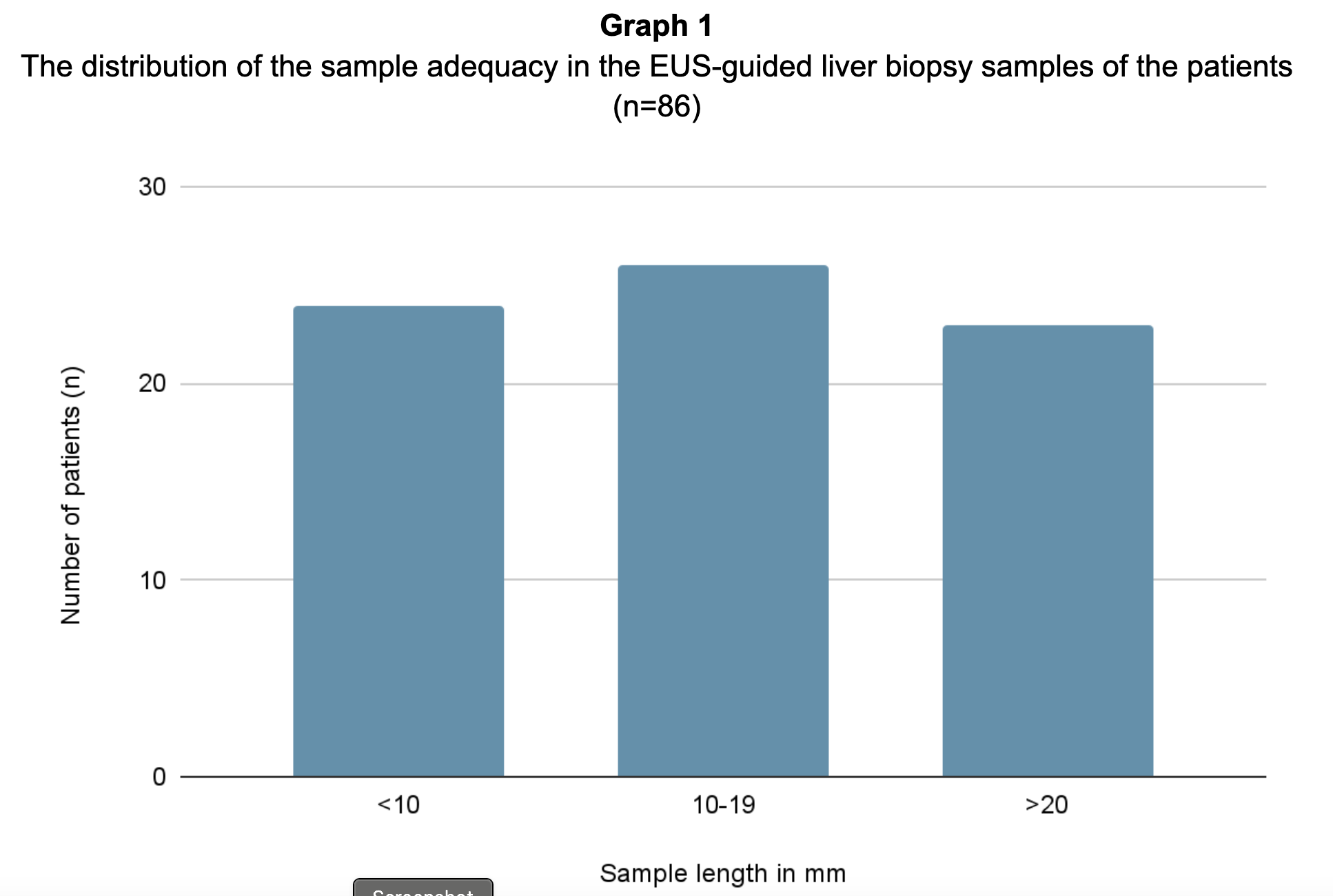
The distribution of the sample adequacy is summarized in Graph 1. Significant pathologies observed in the biopsy samples are summarized in Table 1.
Discussion: EUS-LB in an ASC is feasible and safe. A medium to high volume EUS practice should consider performing EUS-LB in ASC which would add to the revenue for the ASC and could increase access and cost-savings for patients.
Disclosures:
Mankanwal S. Sachdev, MD1, Harshaman Kaur, MD2, Amninder Singh, MD3, Ananya Das, MD4. P4482 - Safety and Feasibility of Endoscopic Ultrasonography Guided Liver Biopsy (EUS-LB) in a Free-Standing Ambulatory Surgery Center (ASC), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1AZCDH, Paradise Valley, AZ; 2Mountain Vista Medical Center, Phoenix, AZ; 3The Wright Center for Graduate Medical Education, Scranton, PA; 4Arizona Centers for Digestive Health, Paradise Valley, AZ
Introduction: EUS has been historically limited to hospital-based facilities due to cost, and resources. ASCs offer convenient, safe, and economical options for the performance of standard endoscopy and are attractive to both patients and healthcare payers because of the provision of high-quality care at a significantly lower cost when compared to hospital-based outpatient facilities (Frakes 2006). In a previous study, we showed that EUS procedures could be safely and efficiently performed in an ASC. As EUS techniques have advanced, the range of procedures and interventions has expanded. EUS-LB has increasingly become a common procedure for endosonographers. In this study, we aimed to show that EUS-LB can be done safely and effectively in an ASC.
Methods: We started to perform EUS-LB in our ASC in 2016, and initially these procedures were done sporadically. Since 2022, EUS-LBs have been done more frequently, and this is a retrospective audit of these cases. All patients, ASA 3 or less, requiring EUS were routinely performed in a free-standing ASC by two experienced endosonographers, in a dedicated EUS room equipped with echoendoscopes. Monthly audits were performed to evaluate adverse events by tracking the average time in the facility (from check-in to check-out) Data was collected from the electronic health record as a part of a quality improvement project.
Results: 86 patients underwent EUS-LB, median age was 58 years (IQR 27), 35% were men, 34% white, and median BMI 30.4 (IQR 10.38). Common comorbidities included hypertension (41.9%) hyperlipidemia (33.7%). 51.2% of patients were obese. The primary indications for biopsy were elevated liver enzymes (55.8%) and abnormal non-invasive liver imaging (14%).
Biopsies were mostly performed on the left lobe (98.8%), using fine-needle biopsy (FNB) exclusively. Needle sizes employed were predominantly 19 G (82.6%), followed by 22 G (12.8%) and 25 G (1.2%). Adverse events were minimal, with abdominal pain reported in 27.9% of cases. There were no occurrences of pneumothorax, hemorrhage, hypotension, tumor seeding, or peritonitis. No patients were hospitalized for the adverse events.
The distribution of the sample adequacy is summarized in Graph 1. Significant pathologies observed in the biopsy samples are summarized in Table 1.
Discussion: EUS-LB in an ASC is feasible and safe. A medium to high volume EUS practice should consider performing EUS-LB in ASC which would add to the revenue for the ASC and could increase access and cost-savings for patients.

Figure: The distribution of the sample adequacy in the EUS-guided liver biopsy samples of the patients (n=86)
Disclosures:
Mankanwal Sachdev: Boston Scientific – Consultant. conmed – Consultant. Fuji – Consultant.
Harshaman Kaur indicated no relevant financial relationships.
Amninder Singh indicated no relevant financial relationships.
Ananya Das: Clear note Health – Consultant.
Mankanwal S. Sachdev, MD1, Harshaman Kaur, MD2, Amninder Singh, MD3, Ananya Das, MD4. P4482 - Safety and Feasibility of Endoscopic Ultrasonography Guided Liver Biopsy (EUS-LB) in a Free-Standing Ambulatory Surgery Center (ASC), ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
