Tuesday Poster Session
Category: Liver
P4638 - Comparative Efficacy of Mycophenolate Mofetil vs Azathioprine as First-Line Treatments for Autoimmune Hepatitis in Treatment Naive Patients: A Meta-Analysis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Muhammad Tayyab Anwar, MD
John H. Stroger, Jr. Hospital of Cook County
Chicago, IL
Presenting Author(s)
Muhammad Tayyab Anwar, MD1, Muhammad Shahzil, MD2, Muhammad Ali Khaqan, MD1, Edzel Lorraine Co, MD3, Alejandro J. Nieto Dominguez, MD1, Sibgha Farooq, MBBS4, Maryam Kausar Nawaz, MBBS5, Rameez Akram Tarar, MD6, Hamza Naeem, MD6, Muhammad Bilal Ibrahim, MD1, Anzal Abdullah, 4
1John H. Stroger, Jr. Hospital of Cook County, Chicago, IL; 2Penn State Health Milton S. Hershey Medical Center, Hershey, PA; 3University of Santo Tomas, Manila, National Capital Region, Philippines; 4University of Health Sciences, Lahore, Punjab, Pakistan; 5Lahore Medical and Dental College, Lahore, Punjab, Pakistan; 6King Edward Medical University, Lahore, Punjab, Pakistan
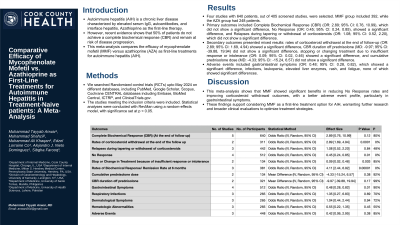
Introduction: Autoimmune hepatitis (AIH) is a chronic liver disease characterized by elevated serum IgG, autoantibodies, and interface hepatitis, Azathioprine as first-line therapy. However, recent evidence shows that 50% of patients do not achieve a complete biochemical response (CBR) and remain at risk of disease progression. This meta-analysis compares the efficacy of mycophenolate mofetil (MMF) versus azathioprine (AZA) as first-line treatments for autoimmune hepatitis (AIH).
Methods: We searched Randomized control trials (RCTs) upto May 2024 on different databases, including PubMed, Google Scholar, Scopus, Cochrane CENTRAL databases including Embase, BioMed Central, ICTRP, and ClinicalTrials.gov. The studies meeting the inclusion criteria were included. Statistical analyses were conducted with RevMan using a random-effects model, with significance set at p < 0.05.
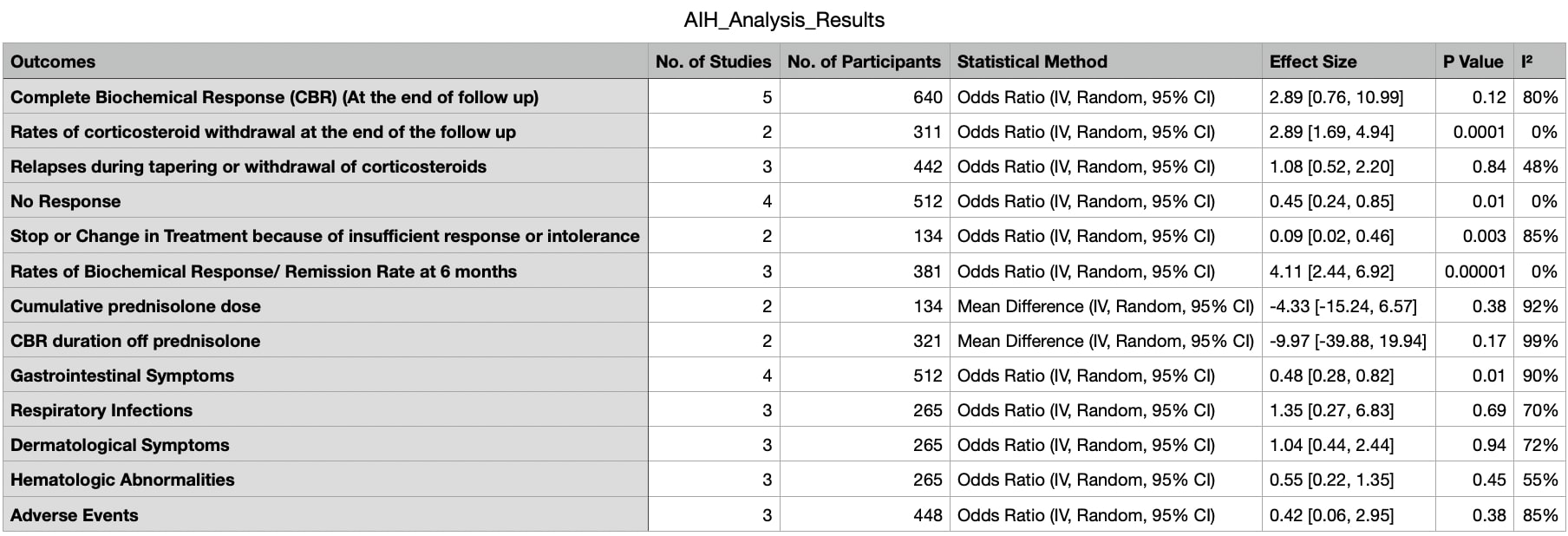
Results: Four studies with 640 patients, out of 405 screened studies, were selected. MMF group included 392, while the AZA group had 248 patients. Primary outcomes included Complete Biochemical Response (CBR) (OR: 2.89; 95% CI: 0.76, 10.99), which did not show a significant difference, No Response (OR: 0.45; 95% CI: 0.24, 0.85), showed a significant difference, and Relapses during tapering or withdrawal of corticosteroids (OR: 1.08; 95% CI: 0.52, 2.20), which did not show a significant difference. Secondary outcomes presented mixed results: rates of corticosteroid withdrawal at the end of follow-up (OR: 2.89; 95% CI: 1.69, 4.94) showed a significant difference, CBR duration off prednisolone (MD: -9.97; 95% CI: -39.88, 19.94) did not show a significant difference, stopping or changing treatment due to insufficient response or intolerance (OR: 0.09; 95% CI: 0.02, 0.46) showed a significant difference, and cumulative prednisolone dose (MD: -4.33; 95% CI: -15.24, 6.57) did not show a significant difference. Adverse events included gastrointestinal symptoms (OR: 0.48; 95% CI: 0.28, 0.82), which showed a significant difference, infections, leukopenia, elevated liver enzymes, rash, and fatigue, none of which showed significant differences.
Discussion: This meta-analysis shows that MMF showed significant benefits in reducing No Response rates and improving corticosteroid withdrawal outcomes, with a better adverse event profile, particularly in gastrointestinal symptoms. These findings support considering MMF as a first-line treatment option for AIH, warranting further research and broader clinical evaluations to optimize treatment strategies.
Disclosures:
Muhammad Tayyab Anwar, MD1, Muhammad Shahzil, MD2, Muhammad Ali Khaqan, MD1, Edzel Lorraine Co, MD3, Alejandro J. Nieto Dominguez, MD1, Sibgha Farooq, MBBS4, Maryam Kausar Nawaz, MBBS5, Rameez Akram Tarar, MD6, Hamza Naeem, MD6, Muhammad Bilal Ibrahim, MD1, Anzal Abdullah, 4. P4638 - Comparative Efficacy of Mycophenolate Mofetil vs Azathioprine as First-Line Treatments for Autoimmune Hepatitis in Treatment Naive Patients: A Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1John H. Stroger, Jr. Hospital of Cook County, Chicago, IL; 2Penn State Health Milton S. Hershey Medical Center, Hershey, PA; 3University of Santo Tomas, Manila, National Capital Region, Philippines; 4University of Health Sciences, Lahore, Punjab, Pakistan; 5Lahore Medical and Dental College, Lahore, Punjab, Pakistan; 6King Edward Medical University, Lahore, Punjab, Pakistan
Introduction: Autoimmune hepatitis (AIH) is a chronic liver disease characterized by elevated serum IgG, autoantibodies, and interface hepatitis, Azathioprine as first-line therapy. However, recent evidence shows that 50% of patients do not achieve a complete biochemical response (CBR) and remain at risk of disease progression. This meta-analysis compares the efficacy of mycophenolate mofetil (MMF) versus azathioprine (AZA) as first-line treatments for autoimmune hepatitis (AIH).
Methods: We searched Randomized control trials (RCTs) upto May 2024 on different databases, including PubMed, Google Scholar, Scopus, Cochrane CENTRAL databases including Embase, BioMed Central, ICTRP, and ClinicalTrials.gov. The studies meeting the inclusion criteria were included. Statistical analyses were conducted with RevMan using a random-effects model, with significance set at p < 0.05.
Results: Four studies with 640 patients, out of 405 screened studies, were selected. MMF group included 392, while the AZA group had 248 patients. Primary outcomes included Complete Biochemical Response (CBR) (OR: 2.89; 95% CI: 0.76, 10.99), which did not show a significant difference, No Response (OR: 0.45; 95% CI: 0.24, 0.85), showed a significant difference, and Relapses during tapering or withdrawal of corticosteroids (OR: 1.08; 95% CI: 0.52, 2.20), which did not show a significant difference. Secondary outcomes presented mixed results: rates of corticosteroid withdrawal at the end of follow-up (OR: 2.89; 95% CI: 1.69, 4.94) showed a significant difference, CBR duration off prednisolone (MD: -9.97; 95% CI: -39.88, 19.94) did not show a significant difference, stopping or changing treatment due to insufficient response or intolerance (OR: 0.09; 95% CI: 0.02, 0.46) showed a significant difference, and cumulative prednisolone dose (MD: -4.33; 95% CI: -15.24, 6.57) did not show a significant difference. Adverse events included gastrointestinal symptoms (OR: 0.48; 95% CI: 0.28, 0.82), which showed a significant difference, infections, leukopenia, elevated liver enzymes, rash, and fatigue, none of which showed significant differences.
Discussion: This meta-analysis shows that MMF showed significant benefits in reducing No Response rates and improving corticosteroid withdrawal outcomes, with a better adverse event profile, particularly in gastrointestinal symptoms. These findings support considering MMF as a first-line treatment option for AIH, warranting further research and broader clinical evaluations to optimize treatment strategies.

Figure: Comparative Efficacy of Mycophenolate Mofetil vs. Azathioprine as First-Line Treatments for Autoimmune Hepatitis: A Meta-Analysis
Disclosures:
Muhammad Tayyab Anwar indicated no relevant financial relationships.
Muhammad Shahzil indicated no relevant financial relationships.
Muhammad Ali Khaqan indicated no relevant financial relationships.
Edzel Lorraine Co indicated no relevant financial relationships.
Alejandro Nieto Dominguez indicated no relevant financial relationships.
Sibgha Farooq indicated no relevant financial relationships.
Maryam Kausar Nawaz indicated no relevant financial relationships.
Rameez Akram Tarar indicated no relevant financial relationships.
Hamza Naeem indicated no relevant financial relationships.
Muhammad Bilal Ibrahim indicated no relevant financial relationships.
Anzal Abdullah indicated no relevant financial relationships.
Muhammad Tayyab Anwar, MD1, Muhammad Shahzil, MD2, Muhammad Ali Khaqan, MD1, Edzel Lorraine Co, MD3, Alejandro J. Nieto Dominguez, MD1, Sibgha Farooq, MBBS4, Maryam Kausar Nawaz, MBBS5, Rameez Akram Tarar, MD6, Hamza Naeem, MD6, Muhammad Bilal Ibrahim, MD1, Anzal Abdullah, 4. P4638 - Comparative Efficacy of Mycophenolate Mofetil vs Azathioprine as First-Line Treatments for Autoimmune Hepatitis in Treatment Naive Patients: A Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
