Tuesday Poster Session
Category: Liver
P4740 - Falling Through The Cracks: A Case Of Cholestatic Injury With Supratherapeutic Testosterone Supplementation
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- MJ
Mikaela Nikkola Jara-Tantoco, MD, MS
Einstein Healthcare Network
Philadelphia, PA
Presenting Author(s)
Mikaela Nikkola Jara-Tantoco, MD, MS1, Nisa Mohammed, MD1, Sana Abbas, MS2, Salvatore Mignano, MD1, Richard Kalman, MD1
1Einstein Healthcare Network, Philadelphia, PA; 2Philadelphia College of Osteopathic Medicine, Philadelphia, PA
Introduction: Drug induced liver injury (DILI) is an adverse reaction to an ingested drug or supplement. Anabolic androgenic steroids (AAS) have been associated with cholestatic DILI. Many individuals use this without a prescription. While patient education is important with the growing use of supplements, it may not be enough with patients who have difficulty navigating the healthcare system.
Case Description/Methods: A 35-year-old healthy African American male presented with a four-month history of painless jaundice, fatigue and malaise. He denied any alcohol or illicit drug use. He visited urgent care centers and a primary care office and was treated with acetaminophen, ibuprofen, amoxicillin/clavulanic acid, and metronidazole. He presented to an outside hospital where he was found to have acute liver injury (ALI) with a right upper quadrant ultrasound showing mildly thickened gallbladder wall without evidence of gallstones or sludge and hepatomegaly. He left against medical advice as he needed to take care of his child.
On admission to our hospital, he had scleral icterus, a nontender abdomen with no palpable masses, and no asterixis. He reported taking AAS, coenzyme Q10, milk thistle, burdock root and dandelion supplements two or more times the recommended daily dose, with AAS preceding symptoms.
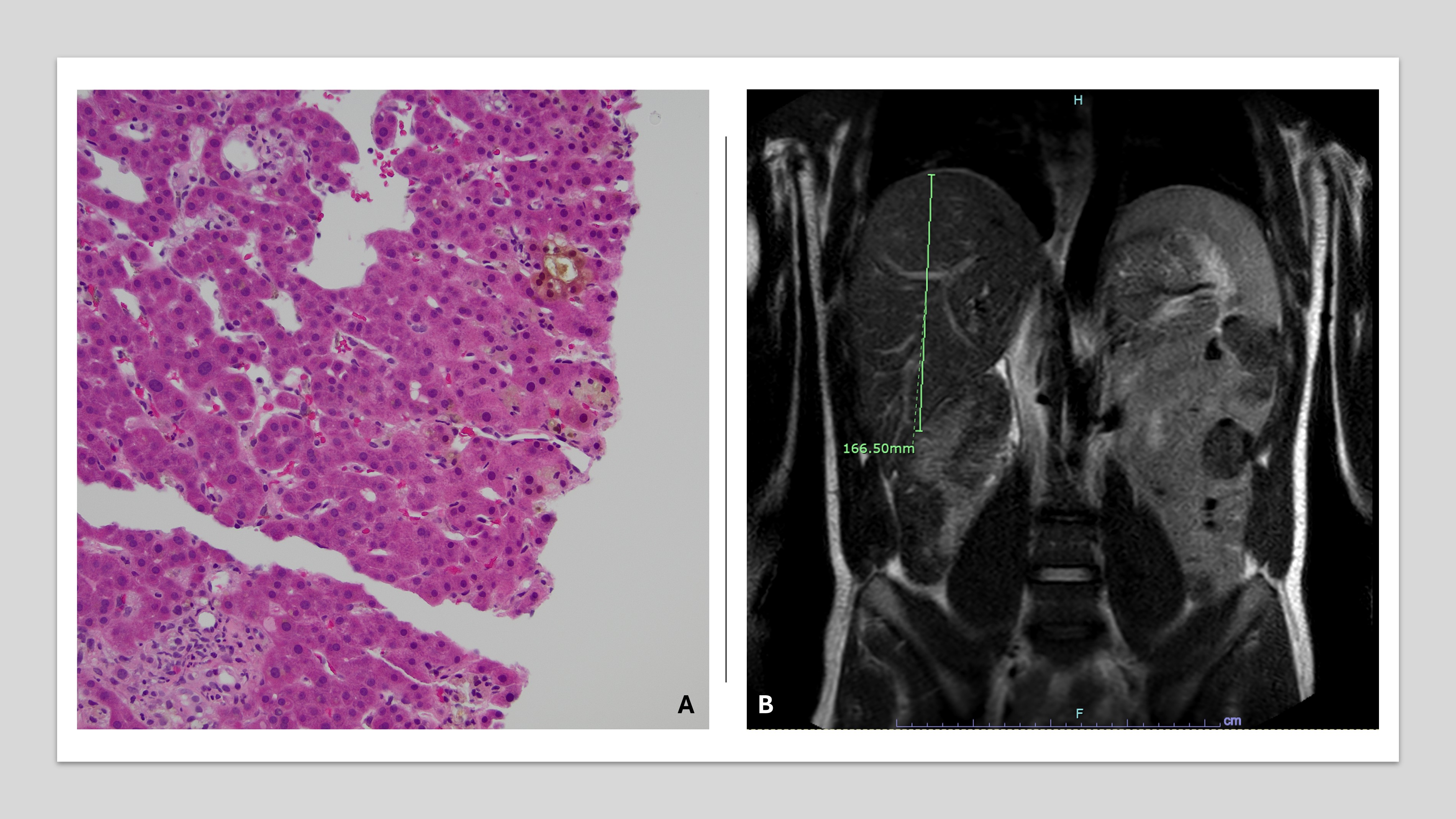
Lab values were significant for alkaline phosphatase of 2,653 units/L (peak 2,928 units/L), total bilirubin 23.3 mg/dL (peak 24.7 mg/dL), direct bilirubin 18.0 mg/dL (peak 18.8 mg/dL), albumin 2.2 g/dL, ALT 237 IU/L and AST 279 IU/L. INR, platelet count, and ammonia level were normal. HIV, ANA, AMA, anti-SMA, EBV, CMV, ceruloplasmin, alcohol, and urine drug screen were negative. MRCP shown in figure.
R ratio was determined to be 0.23 and Roussel Uclaf Causality Assessment Method was +4. The liver biopsy was consistent with cholestatic injury with no areas of necrosis.
He was initiated on ursodiol 300 mg twice a day with improvement of laboratory parameters. He was discharged with an outpatient hepatology follow up appointment. Unfortunately, the patient did not follow-up with his set clinic appointment and instead returned to the emergency room.
Discussion: DILI remains a major cause of ALI in the United States. AAS is often used off-label without physician consultation. Our patient had socioeconomic issues that hindered him from seeking appropriate care, despite continued patient education. Difficulty navigating the healthcare system can aggravate DILI, especially with mixed supratherapeutic use of supplements.
Disclosures:
Mikaela Nikkola Jara-Tantoco, MD, MS1, Nisa Mohammed, MD1, Sana Abbas, MS2, Salvatore Mignano, MD1, Richard Kalman, MD1. P4740 - Falling Through The Cracks: A Case Of Cholestatic Injury With Supratherapeutic Testosterone Supplementation, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Einstein Healthcare Network, Philadelphia, PA; 2Philadelphia College of Osteopathic Medicine, Philadelphia, PA
Introduction: Drug induced liver injury (DILI) is an adverse reaction to an ingested drug or supplement. Anabolic androgenic steroids (AAS) have been associated with cholestatic DILI. Many individuals use this without a prescription. While patient education is important with the growing use of supplements, it may not be enough with patients who have difficulty navigating the healthcare system.
Case Description/Methods: A 35-year-old healthy African American male presented with a four-month history of painless jaundice, fatigue and malaise. He denied any alcohol or illicit drug use. He visited urgent care centers and a primary care office and was treated with acetaminophen, ibuprofen, amoxicillin/clavulanic acid, and metronidazole. He presented to an outside hospital where he was found to have acute liver injury (ALI) with a right upper quadrant ultrasound showing mildly thickened gallbladder wall without evidence of gallstones or sludge and hepatomegaly. He left against medical advice as he needed to take care of his child.
On admission to our hospital, he had scleral icterus, a nontender abdomen with no palpable masses, and no asterixis. He reported taking AAS, coenzyme Q10, milk thistle, burdock root and dandelion supplements two or more times the recommended daily dose, with AAS preceding symptoms.
Lab values were significant for alkaline phosphatase of 2,653 units/L (peak 2,928 units/L), total bilirubin 23.3 mg/dL (peak 24.7 mg/dL), direct bilirubin 18.0 mg/dL (peak 18.8 mg/dL), albumin 2.2 g/dL, ALT 237 IU/L and AST 279 IU/L. INR, platelet count, and ammonia level were normal. HIV, ANA, AMA, anti-SMA, EBV, CMV, ceruloplasmin, alcohol, and urine drug screen were negative. MRCP shown in figure.
R ratio was determined to be 0.23 and Roussel Uclaf Causality Assessment Method was +4. The liver biopsy was consistent with cholestatic injury with no areas of necrosis.
He was initiated on ursodiol 300 mg twice a day with improvement of laboratory parameters. He was discharged with an outpatient hepatology follow up appointment. Unfortunately, the patient did not follow-up with his set clinic appointment and instead returned to the emergency room.
Discussion: DILI remains a major cause of ALI in the United States. AAS is often used off-label without physician consultation. Our patient had socioeconomic issues that hindered him from seeking appropriate care, despite continued patient education. Difficulty navigating the healthcare system can aggravate DILI, especially with mixed supratherapeutic use of supplements.

Figure: A. The image shows features of cholestatic injury, including bile plugging and bile ductular reaction. Pigmented macrophages are also present. HE, x200.
B. MRI of the abdomen without IV contrast, utilizing standard MRCP protocol: The liver is normal in signal intensity, top normal in size, and has a smooth contour. There is no signal dropout on opposed phase images to suggest steatosis. Normal appearance of the biliary tree with no choledocholithiasis or biliary ductal dilatation. No pancreatic mass identified.
B. MRI of the abdomen without IV contrast, utilizing standard MRCP protocol: The liver is normal in signal intensity, top normal in size, and has a smooth contour. There is no signal dropout on opposed phase images to suggest steatosis. Normal appearance of the biliary tree with no choledocholithiasis or biliary ductal dilatation. No pancreatic mass identified.
Disclosures:
Mikaela Nikkola Jara-Tantoco indicated no relevant financial relationships.
Nisa Mohammed indicated no relevant financial relationships.
Sana Abbas indicated no relevant financial relationships.
Salvatore Mignano indicated no relevant financial relationships.
Richard Kalman indicated no relevant financial relationships.
Mikaela Nikkola Jara-Tantoco, MD, MS1, Nisa Mohammed, MD1, Sana Abbas, MS2, Salvatore Mignano, MD1, Richard Kalman, MD1. P4740 - Falling Through The Cracks: A Case Of Cholestatic Injury With Supratherapeutic Testosterone Supplementation, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
