Tuesday Poster Session
Category: Liver
P4787 - A Case of Successful Treatment of High-Grade Immune-Mediated Hepatobiliary Toxicity With Oral Budesonide: Shifting the Paradigm Away From Systemic Steroids
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Hao Chi Zhang, MD
MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
Award: Presidential Poster Award
Hao Chi Zhang, MD1, Ethan Miller, MD1, Lan Wang, MD2
1MD Anderson Cancer Center, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: For the treatment of high-grade immune-mediated hepatotoxicity (IMH), oncology societies' guidance documents have endorsed administering high-dose systemic steroids (1-2 mg/kg/d prednisone-equivalent). Recent clinical experiences have questioned the necessity of this approach and have introduced the notion of utilizing oral budesonide, instead. We present a case in which high-grade IMH was managed with budesonide as the only steroid, along with ursodiol, yielding successful complete biochemical remission.
Case Description/Methods: A 66-year-old man with a history of recurrent squamous cell carcinoma of the oral cavity treated by resection and with 5 cycles of carboplatin/paclitaxel/pembrolizumab as treatment. He had no pre-existing chronic liver disease or alcohol use. About 1.5 months after his last dose of pembrolizumab, he developed abnormal liver biochemistries including ALT 1582 U/L (45×ULN, CTCAE grade 4), AST 1132 U/L, alkaline phosphatase (ALP) 1036 U/L, GGT 437 U/L, total bilirubin 3.3 mg/dL (direct bilirubin 2.5 mg/dL), and INR 1.03. On physical exam, he did not exhibit jaundice, abnormal mentation, or abdominal tenderness. Oral budesonide 9 mg/d and ursodiol 1200 mg/d were initiated for suspected anti-PD-1-induced IMH. Comprehensive serologic testing for liver disease and liver imaging were unrevealing. Liver biopsy performed 4 days into admission showed moderate acute lobular/portal lymphocytic hepatitis with spotty necrosis and mild bile duct injury, compatible with IMH. Budesonide was continued for 25 days until ALT was < 90 U/L, and a taper of budesonide to 6 mg/d and 3 mg/d (2 weeks each) was completed. Biochemical remission was achieved after 35 days of budesonide therapy. Total duration of budesonide was 53 days (1.8 months); total duration of ursodiol was 2.9 months. Pembrolizumab was not resumed, and the patient moved on to radiation treatment.
Discussion: To treat classic idiopathic autoimmune hepatitis, prednisone (up to 60 mg/d) or budesonide (9 mg/d) are established options for initial therapy. We demonstrate that even CTCAE grade 4 IMH can also be effectively managed using budesonide, without need for systemic steroids, to avert steroid-related adverse effects. One case report by Eleftheriotis et al. (2022) and a recent abstract by Pizuorno Machado et al. (2023) (N=16) showed excellent success with using oral budesonide to treat IMH. Additional studies with budesonide should be explored in this field to advance understanding of optimal therapies for IMH.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Hao Chi Zhang, MD1, Ethan Miller, MD1, Lan Wang, MD2. P4787 - A Case of Successful Treatment of High-Grade Immune-Mediated Hepatobiliary Toxicity With Oral Budesonide: Shifting the Paradigm Away From Systemic Steroids, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
Hao Chi Zhang, MD1, Ethan Miller, MD1, Lan Wang, MD2
1MD Anderson Cancer Center, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: For the treatment of high-grade immune-mediated hepatotoxicity (IMH), oncology societies' guidance documents have endorsed administering high-dose systemic steroids (1-2 mg/kg/d prednisone-equivalent). Recent clinical experiences have questioned the necessity of this approach and have introduced the notion of utilizing oral budesonide, instead. We present a case in which high-grade IMH was managed with budesonide as the only steroid, along with ursodiol, yielding successful complete biochemical remission.
Case Description/Methods: A 66-year-old man with a history of recurrent squamous cell carcinoma of the oral cavity treated by resection and with 5 cycles of carboplatin/paclitaxel/pembrolizumab as treatment. He had no pre-existing chronic liver disease or alcohol use. About 1.5 months after his last dose of pembrolizumab, he developed abnormal liver biochemistries including ALT 1582 U/L (45×ULN, CTCAE grade 4), AST 1132 U/L, alkaline phosphatase (ALP) 1036 U/L, GGT 437 U/L, total bilirubin 3.3 mg/dL (direct bilirubin 2.5 mg/dL), and INR 1.03. On physical exam, he did not exhibit jaundice, abnormal mentation, or abdominal tenderness. Oral budesonide 9 mg/d and ursodiol 1200 mg/d were initiated for suspected anti-PD-1-induced IMH. Comprehensive serologic testing for liver disease and liver imaging were unrevealing. Liver biopsy performed 4 days into admission showed moderate acute lobular/portal lymphocytic hepatitis with spotty necrosis and mild bile duct injury, compatible with IMH. Budesonide was continued for 25 days until ALT was < 90 U/L, and a taper of budesonide to 6 mg/d and 3 mg/d (2 weeks each) was completed. Biochemical remission was achieved after 35 days of budesonide therapy. Total duration of budesonide was 53 days (1.8 months); total duration of ursodiol was 2.9 months. Pembrolizumab was not resumed, and the patient moved on to radiation treatment.
Discussion: To treat classic idiopathic autoimmune hepatitis, prednisone (up to 60 mg/d) or budesonide (9 mg/d) are established options for initial therapy. We demonstrate that even CTCAE grade 4 IMH can also be effectively managed using budesonide, without need for systemic steroids, to avert steroid-related adverse effects. One case report by Eleftheriotis et al. (2022) and a recent abstract by Pizuorno Machado et al. (2023) (N=16) showed excellent success with using oral budesonide to treat IMH. Additional studies with budesonide should be explored in this field to advance understanding of optimal therapies for IMH.

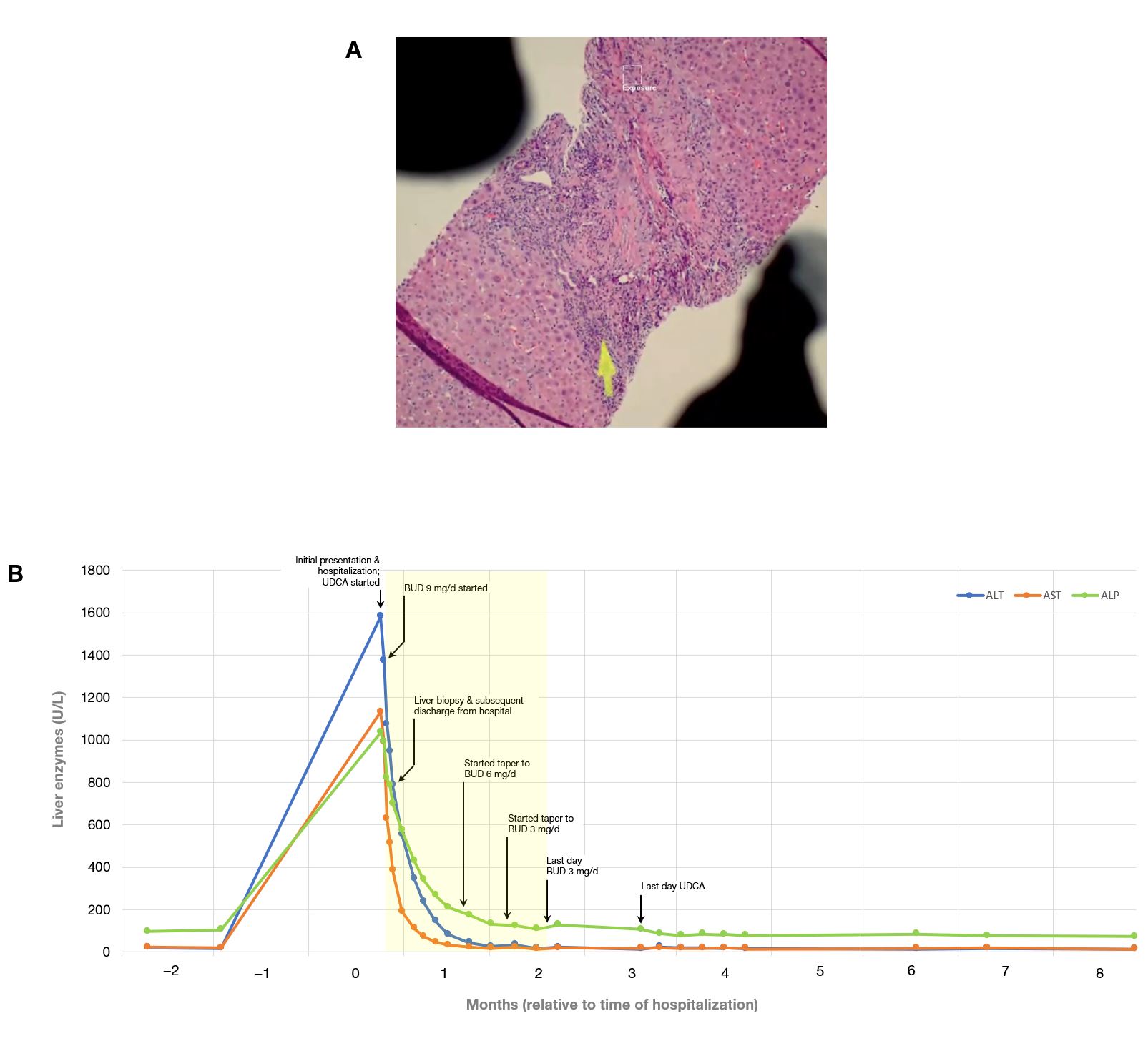
Figure: Panel A: Liver biopsy (hematoxylin & eosin stain): histology demonstrating moderate acute lobular and portal hepatitis with combination of lymphocytes and neutrophils, with portal edema and interface hepatitis, but without appreciable plasma cells (arrow); bile duct injury was deemed mild, without bile stasis; no significant steatosis or other features of chronic liver disease. In the clinical context, the histology was deemed compatible with liver injury associated with immune checkpoint inhibitor. | Panel B: Chronological trends of liver biochemical labs during the clinical course and with respect to initial presentation or hospitalization and treatments prescribed. The biochemical of ALP elevation was seemingly out of proportion to findings of only mild bile duct injury, but was associated with mild bilirubin elevation without liver dysfunction. Budesonide 9 mg/d was initiated, without need for systemic steroids. UDCA was simultaneously prescribed as an upfront adjunct due to the cholangiopathic features, albeit mild histologically. Biochemical remission was achieved at about 35 d into the budesonide course. The light yellow region corresponds to the period during which the patient took budesonide (including the taper phases). UDCA was discontinued after attaining normal values of ALP and GGT.
Abbreviations: IMH, immune-mediated hepatobiliary toxicity; BUD, budesonide; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; d, day; IV, intravenous; MP, methylprednisolone; pred., prednisone; UDCA, ursodeoxycholic acid.
Abbreviations: IMH, immune-mediated hepatobiliary toxicity; BUD, budesonide; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; d, day; IV, intravenous; MP, methylprednisolone; pred., prednisone; UDCA, ursodeoxycholic acid.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Hao Chi Zhang indicated no relevant financial relationships.
Ethan Miller indicated no relevant financial relationships.
Lan Wang indicated no relevant financial relationships.
Hao Chi Zhang, MD1, Ethan Miller, MD1, Lan Wang, MD2. P4787 - A Case of Successful Treatment of High-Grade Immune-Mediated Hepatobiliary Toxicity With Oral Budesonide: Shifting the Paradigm Away From Systemic Steroids, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.


