Tuesday Poster Session
Category: Liver
P4830 - A Case of Amatoxin-Induced Acute Liver Failure in Central Massachusetts
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Mary Bassaly, MD
University of Massachusetts Chan Medical School, MA
Presenting Author(s)
Mary Bassaly, MD1, Kristen Moulton, DO2, Navine Nasser-Ghodsi, MD3, Savant Mehta, MBBS, MD, DM4
1University of Massachusetts Chan Medical School, Worcester, MA; 2University of Massachusetts Memorial Medical Center, Worcester, MA; 3UMass Chan Medical School & UMass Memorial Health Care, Worcester, MA; 4UMASS Memorial Healthcare, Worcester, MA
Introduction: Amatoxin mushrooms are a rare cause of acute liver failure (ALF). Management includes medical stabilization, blocking absorption, and preventing hepatocyte uptake. This case highlights two less common treatments of amatoxin-induced ALF at an academic medical center.
Case Description/Methods: A 63-year-old female with chronic kidney disease presented to a community hospital with abdominal pain, vomiting, and diarrhea after ingesting foraged mushrooms two days prior. Initial liver enzymes showed aspartate aminotransferase of 135 U/L, alanine transaminase of 73 U/L, total bilirubin of 1.7 mg/dL, and international normalized ratio (INR) of 2.0. Within 24 hours she was diagnosed with ALF evidenced by encephalopathy (West Haven (WH) grade 1), worsening liver tests ( >6x initial values), and coagulopathy (INR 4.2). Toxicologists recommended transfer for a liver transplant (LT) evaluation. The Food and Drug Administration (FDA) approved emergency use of intravenous (IV) silibinin.
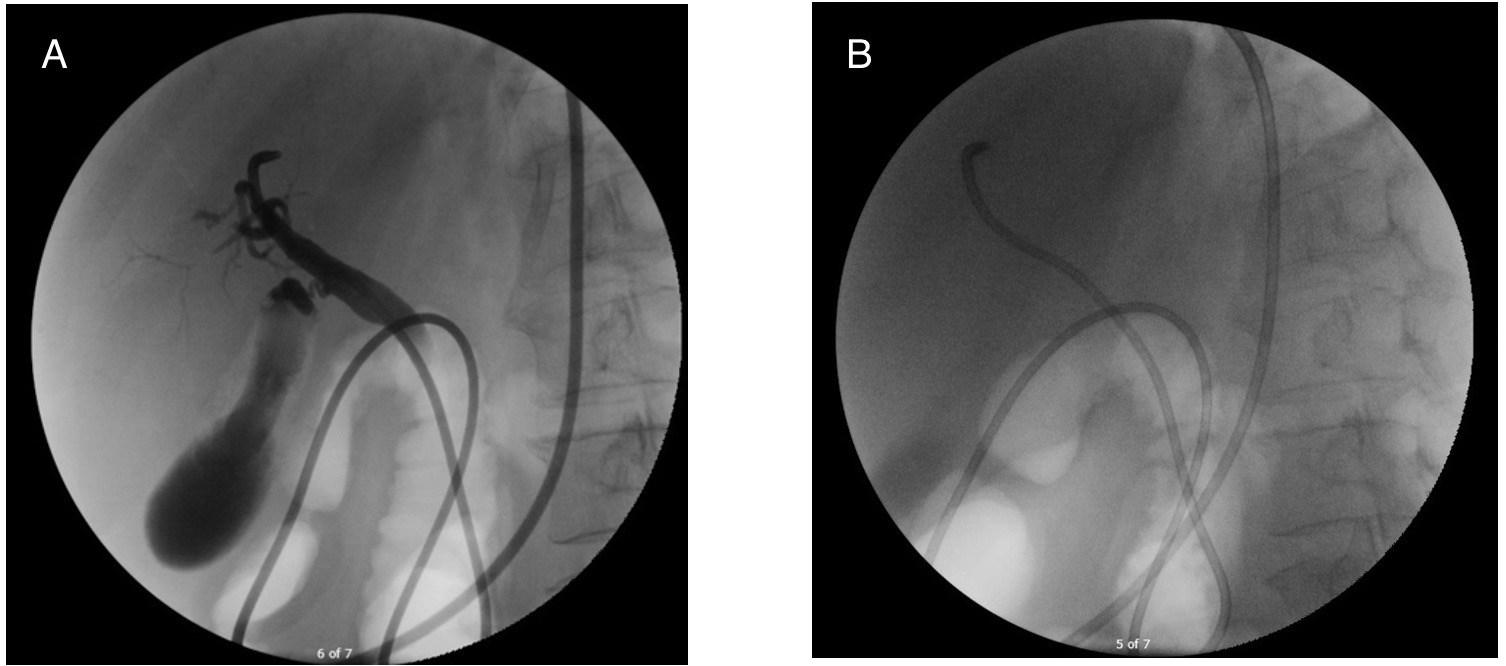
On arrival, her Model for End-Stage Liver Disease (MELD) was 39. An expedited LT evaluation was initiated. She received IV silibinin, IV octreotide, IV N-acetyl cysteine, and high dose penicillin. She underwent a nasobiliary drain via endoscopic retrograde cholangiopancreatography (Figure 1); the drain had minimal output despite confirmed placement.
The following day, the patient’s encephalopathy progressed to WH grade 3. She had acute on chronic renal failure requiring renal replacement therapy. She was listed for LT and underwent a deceased donor LT two days later.
Discussion: Amatoxin inhibits ribonucleic acid (RNA) polymerase, preventing messenger RNA transcription in hepatocytes, causing nucleoli disintegration and hepatic necrosis. It can progress to ALF within 48 hours.
Silibinin prevents uptake of amatoxin in hepatocytes. It is most effective when initiated close to time of ingestion. The community hospital sought FDA approval thus expediting its initiation after transfer, likely helping to stabilize the patient prior to LT.
A nasobiliary drain removes amatoxin from the bile and is most helpful within 24 hours of presentation. Although successfully placed, she did not have significant biliary drain output. Nasobiliary drain placement is an uncommon procedure likely limited to tertiary medical centers.
Prompt transfer to a LT center in patients with acute liver injury due to suspected amatoxin poisoning allows for optimal medical and endoscopic detoxification, in addition to an expedited LT evaluation.
Disclosures:
Mary Bassaly, MD1, Kristen Moulton, DO2, Navine Nasser-Ghodsi, MD3, Savant Mehta, MBBS, MD, DM4. P4830 - A Case of Amatoxin-Induced Acute Liver Failure in Central Massachusetts, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Massachusetts Chan Medical School, Worcester, MA; 2University of Massachusetts Memorial Medical Center, Worcester, MA; 3UMass Chan Medical School & UMass Memorial Health Care, Worcester, MA; 4UMASS Memorial Healthcare, Worcester, MA
Introduction: Amatoxin mushrooms are a rare cause of acute liver failure (ALF). Management includes medical stabilization, blocking absorption, and preventing hepatocyte uptake. This case highlights two less common treatments of amatoxin-induced ALF at an academic medical center.
Case Description/Methods: A 63-year-old female with chronic kidney disease presented to a community hospital with abdominal pain, vomiting, and diarrhea after ingesting foraged mushrooms two days prior. Initial liver enzymes showed aspartate aminotransferase of 135 U/L, alanine transaminase of 73 U/L, total bilirubin of 1.7 mg/dL, and international normalized ratio (INR) of 2.0. Within 24 hours she was diagnosed with ALF evidenced by encephalopathy (West Haven (WH) grade 1), worsening liver tests ( >6x initial values), and coagulopathy (INR 4.2). Toxicologists recommended transfer for a liver transplant (LT) evaluation. The Food and Drug Administration (FDA) approved emergency use of intravenous (IV) silibinin.
On arrival, her Model for End-Stage Liver Disease (MELD) was 39. An expedited LT evaluation was initiated. She received IV silibinin, IV octreotide, IV N-acetyl cysteine, and high dose penicillin. She underwent a nasobiliary drain via endoscopic retrograde cholangiopancreatography (Figure 1); the drain had minimal output despite confirmed placement.
The following day, the patient’s encephalopathy progressed to WH grade 3. She had acute on chronic renal failure requiring renal replacement therapy. She was listed for LT and underwent a deceased donor LT two days later.
Discussion: Amatoxin inhibits ribonucleic acid (RNA) polymerase, preventing messenger RNA transcription in hepatocytes, causing nucleoli disintegration and hepatic necrosis. It can progress to ALF within 48 hours.
Silibinin prevents uptake of amatoxin in hepatocytes. It is most effective when initiated close to time of ingestion. The community hospital sought FDA approval thus expediting its initiation after transfer, likely helping to stabilize the patient prior to LT.
A nasobiliary drain removes amatoxin from the bile and is most helpful within 24 hours of presentation. Although successfully placed, she did not have significant biliary drain output. Nasobiliary drain placement is an uncommon procedure likely limited to tertiary medical centers.
Prompt transfer to a LT center in patients with acute liver injury due to suspected amatoxin poisoning allows for optimal medical and endoscopic detoxification, in addition to an expedited LT evaluation.

Figure: Figure 1 (A and B): Fluoroscopic images, obtained during endoscopic retrograde cholangiopancreatography (ERCP) and placement of nasobiliary drain.
Disclosures:
Mary Bassaly indicated no relevant financial relationships.
Kristen Moulton indicated no relevant financial relationships.
Navine Nasser-Ghodsi: Boston Scientific – Consultant.
Savant Mehta indicated no relevant financial relationships.
Mary Bassaly, MD1, Kristen Moulton, DO2, Navine Nasser-Ghodsi, MD3, Savant Mehta, MBBS, MD, DM4. P4830 - A Case of Amatoxin-Induced Acute Liver Failure in Central Massachusetts, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
