Tuesday Poster Session
Category: Liver
P4854 - Lys166Asn HFE Variant Mutation Causing Hemochromatosis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Edward Cay, DO
Rush University Medical Center, MI
Presenting Author(s)
Edward Cay, DO1, Samantha Crosby, MS2, Daniel Douce, MD2
1Rush University Medical Center, Chicago, IL; 2Corewell Health, St. Joseph, MI
Introduction: Hereditary hemochromatosis (HH) is an autosomal recessive condition characterized by iron overload causing organ damage, such as cirrhosis, congestive heart failure, diabetes and hypogonadism. We present a patient who required phlebotomy due to iron overload, despite testing only heterozygous for C282Y but also for a K166N variant.
Case Description/Methods: A 49-year-old man was found to have a ferritin of 1,593 ng/dL as part of an occupational health physical. While his family was of Northern European/ Baltic German descent, he had no known family history of hemochromatosis, nor personal history of liver disease. Other labs were significant for hemoglobin of 15.5 g/dL, iron saturation >95%, transferrin saturation 92% and unremarkable liver chemistries.
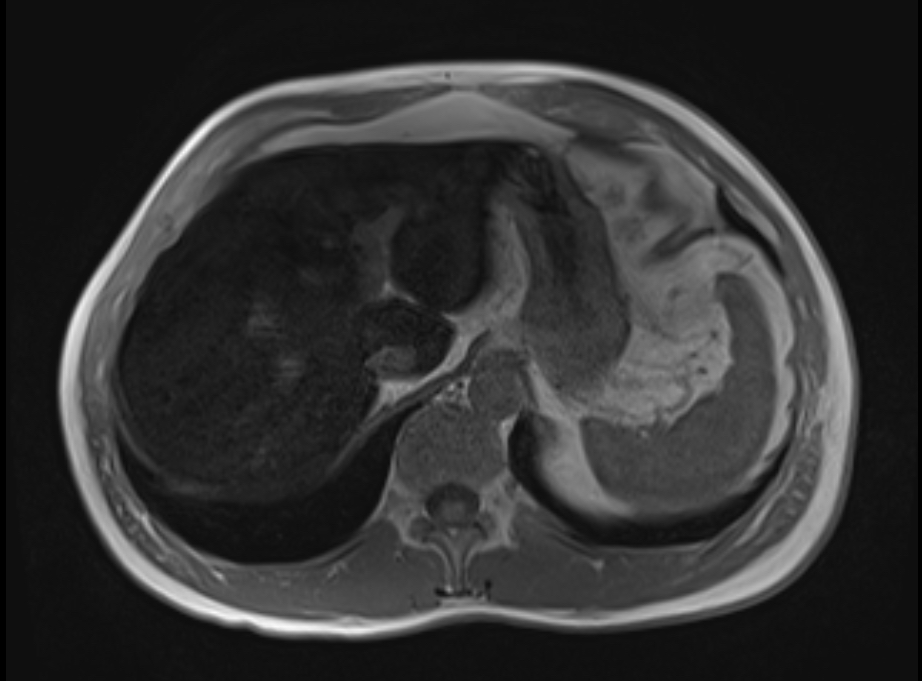
Initial genetic testing for HH returned heterozygous for C282Y and negative for H63D. Due to a high index of suspicion for hemochromatosis, magnetic resonance imaging was ordered, showed marked loss of signal on imaging within the liver compatible with iron deposition, with normal size and smooth surface.
Expanded genetic testing revealed a variant of uncertain significance, K166N, within the HFE gene. Consequently, serial weekly 500 cc phlebotomies were scheduled due to suspicion of an uncommon variant of hemochromatosis, which he tolerated well.
Discussion: Gene mutations of the homeostatic iron regulator (HFE) protein lead to decreased hepcidin expression, promoting ferroportin to increase iron absorption. While homozygosity of the C282Y mutation of the HFE gene causes HH, heterozygosity of C282Y typically express only a carrier phenotype.
However, there have been rare variants of undetermined significance (VUS) noted on a different part of the HFE gene on expanded testing which has been observed in patients with hemochromatosis. These patients were heterozygous for C282Y as well as for a VUS.
The K166N missense mutation found in our patient replaces the amino acid lysine, which is basic and polar, to asparagine, which is neutral and polar. This change occurs at codon 166 of the HFE protein, affecting its function at the alpha 2 domain and leading to a conformational change of the protein that hinders its functioning.
In conclusion, full gene sequencing of HFE may be reasonable to perform in C282Y heterozygous patient in whom there is a high index of suspicion for iron overload.
Disclosures:
Edward Cay, DO1, Samantha Crosby, MS2, Daniel Douce, MD2. P4854 - Lys166Asn HFE Variant Mutation Causing Hemochromatosis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Rush University Medical Center, Chicago, IL; 2Corewell Health, St. Joseph, MI
Introduction: Hereditary hemochromatosis (HH) is an autosomal recessive condition characterized by iron overload causing organ damage, such as cirrhosis, congestive heart failure, diabetes and hypogonadism. We present a patient who required phlebotomy due to iron overload, despite testing only heterozygous for C282Y but also for a K166N variant.
Case Description/Methods: A 49-year-old man was found to have a ferritin of 1,593 ng/dL as part of an occupational health physical. While his family was of Northern European/ Baltic German descent, he had no known family history of hemochromatosis, nor personal history of liver disease. Other labs were significant for hemoglobin of 15.5 g/dL, iron saturation >95%, transferrin saturation 92% and unremarkable liver chemistries.
Initial genetic testing for HH returned heterozygous for C282Y and negative for H63D. Due to a high index of suspicion for hemochromatosis, magnetic resonance imaging was ordered, showed marked loss of signal on imaging within the liver compatible with iron deposition, with normal size and smooth surface.
Expanded genetic testing revealed a variant of uncertain significance, K166N, within the HFE gene. Consequently, serial weekly 500 cc phlebotomies were scheduled due to suspicion of an uncommon variant of hemochromatosis, which he tolerated well.
Discussion: Gene mutations of the homeostatic iron regulator (HFE) protein lead to decreased hepcidin expression, promoting ferroportin to increase iron absorption. While homozygosity of the C282Y mutation of the HFE gene causes HH, heterozygosity of C282Y typically express only a carrier phenotype.
However, there have been rare variants of undetermined significance (VUS) noted on a different part of the HFE gene on expanded testing which has been observed in patients with hemochromatosis. These patients were heterozygous for C282Y as well as for a VUS.
The K166N missense mutation found in our patient replaces the amino acid lysine, which is basic and polar, to asparagine, which is neutral and polar. This change occurs at codon 166 of the HFE protein, affecting its function at the alpha 2 domain and leading to a conformational change of the protein that hinders its functioning.
In conclusion, full gene sequencing of HFE may be reasonable to perform in C282Y heterozygous patient in whom there is a high index of suspicion for iron overload.

Figure: Magnetic resonance imaging of liver demonstrating marked loss of signal on in phase imaging consistent with iron deposition.
Disclosures:
Edward Cay indicated no relevant financial relationships.
Samantha Crosby indicated no relevant financial relationships.
Daniel Douce indicated no relevant financial relationships.
Edward Cay, DO1, Samantha Crosby, MS2, Daniel Douce, MD2. P4854 - Lys166Asn HFE Variant Mutation Causing Hemochromatosis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
