Tuesday Poster Session
Category: Obesity
P4882 - Association of GLP-1 RA Or Dual GIP and GLP-1 RA for Obesity And Gastrointestinal Adverse Events: A Systematic Review
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Lizeth Cifuentes, MD
University of Pittsburgh Medical Center
Pittsburgh, PA
Presenting Author(s)
Lizeth Cifuentes, MD1, Mehdi Abbasi, MD2, Wissam Ghusn, MD3, Domenica Jijon, MD4, John Navarrete, MD4, Mayte Insuasti, MD4, Joseph M.. Murphy, MD4, Nanditha Venkatesan, MD1, Shakira Yoosuf, MD1, Anusha Amaravathi, MD1, Mateo Fabara, MD4
1University of Pittsburgh Medical Center, Pittsburgh, PA; 2University of New Mexico Hospital, New Mexico, NM; 3Boston University Medical Center, Boston, MA; 4Universidad de Las Américas, Quito, Pichincha, Ecuador
Introduction: The emergence of glucagon-like peptide 1 (GLP-1) receptor agonists and the GLP-1/glucose-dependent insulinotropic polypeptide (GLP-1/GIP) coagonist, tirzepatide, marks a revolutionary era in obesity management. However, the novel nature of these drugs necessitates a meticulous examination of potential adverse effects, particularly on the gastrointestinal system, which may limit their use.
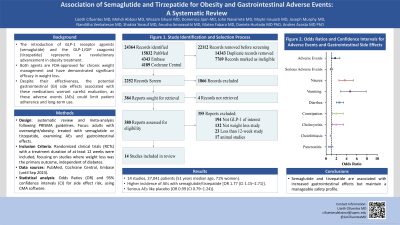
Methods: Following PRISMA guidelines, we systematically reviewed randomized clinical trials (RCTs) conducted among adults with overweight and obesity treated with liraglutide, semaglutide, and tirzepatide for at least 12 weeks, with weight loss as the primary outcome, irrespective of diabetes status, compared with another active agent or placebo. A comprehensive search was performed across PubMed, Cochrane Central, and Embase from inception to September 15, 2023. Our objective was to evaluate the incidence of adverse events (AEs) and serious AEs leading to treatment discontinuation, along with the total number and proportion of gastrointestinal events. Odds ratios (OR) with 95% confidence intervals (CI) were employed for medication-related side effect risk analysis. All statistical analyses were conducted using CMA software. The study is registered in PROSPERO (CRD42023458537).
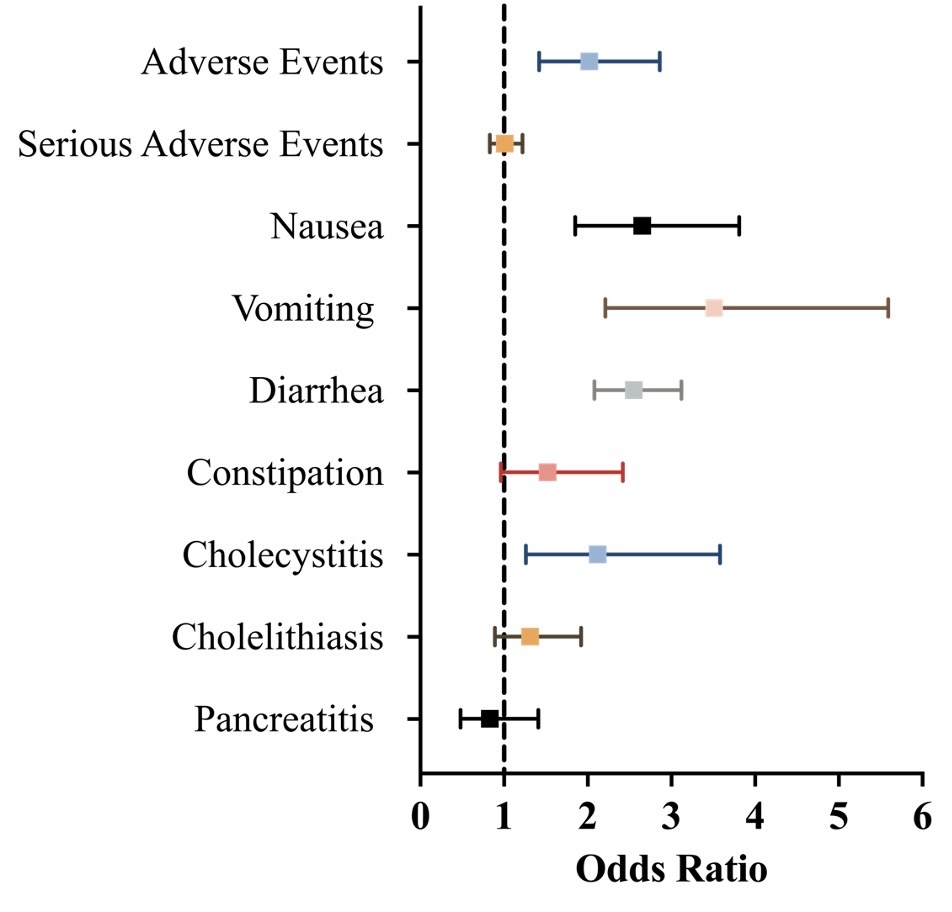
Results: Our analysis encompassed 25 studies, involving 31,826 patients (median age: 51.6 years; 56% women; median baseline body weight: 98.8 kg; median baseline body mass index: 35.4). The GLP-1/GLP-1/GIP group exhibited a higher incidence of AEs compared to the control group [OR 2.02 (95% CI 1.42 to 2.86)]. However, the number of serious AEs did not differ significantly between the GLP-1/GLP-1/GIP group and placebo groups [OR 1.01 (95% CI 0.83 to 1.22)]. Specifically, gastrointestinal side effects were notable in the GLP-1/GLP-1/GIP group. Nausea [OR 2.65 (95% CI 1.85 to 3.81, N=19)], vomiting [OR 3.51 (95% CI 2.21 to 5.59, N=19)], diarrhea [OR 2.55 (95% CI 2.08 to 3.12, N=18)], and cholecystitis [OR 2.12 (95% CI 1.26 to 3.58, N=15)] were significantly higher compared with placebo. Conversely, the rates of constipation [OR 1.52 (95% CI 0.96 to 2.42, N=18)], cholelithiasis [OR 1.31 (95% CI 0.89 to 1.92, N=15)], and pancreatitis [OR 0.83 (95% CI 0.48 to 1.41, N=9)] were comparable between the two groups.
Discussion:
Disclosures:
Lizeth Cifuentes, MD1, Mehdi Abbasi, MD2, Wissam Ghusn, MD3, Domenica Jijon, MD4, John Navarrete, MD4, Mayte Insuasti, MD4, Joseph M.. Murphy, MD4, Nanditha Venkatesan, MD1, Shakira Yoosuf, MD1, Anusha Amaravathi, MD1, Mateo Fabara, MD4. P4882 - Association of GLP-1 RA Or Dual GIP and GLP-1 RA for Obesity And Gastrointestinal Adverse Events: A Systematic Review, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Pittsburgh Medical Center, Pittsburgh, PA; 2University of New Mexico Hospital, New Mexico, NM; 3Boston University Medical Center, Boston, MA; 4Universidad de Las Américas, Quito, Pichincha, Ecuador
Introduction: The emergence of glucagon-like peptide 1 (GLP-1) receptor agonists and the GLP-1/glucose-dependent insulinotropic polypeptide (GLP-1/GIP) coagonist, tirzepatide, marks a revolutionary era in obesity management. However, the novel nature of these drugs necessitates a meticulous examination of potential adverse effects, particularly on the gastrointestinal system, which may limit their use.
Methods: Following PRISMA guidelines, we systematically reviewed randomized clinical trials (RCTs) conducted among adults with overweight and obesity treated with liraglutide, semaglutide, and tirzepatide for at least 12 weeks, with weight loss as the primary outcome, irrespective of diabetes status, compared with another active agent or placebo. A comprehensive search was performed across PubMed, Cochrane Central, and Embase from inception to September 15, 2023. Our objective was to evaluate the incidence of adverse events (AEs) and serious AEs leading to treatment discontinuation, along with the total number and proportion of gastrointestinal events. Odds ratios (OR) with 95% confidence intervals (CI) were employed for medication-related side effect risk analysis. All statistical analyses were conducted using CMA software. The study is registered in PROSPERO (CRD42023458537).
Results: Our analysis encompassed 25 studies, involving 31,826 patients (median age: 51.6 years; 56% women; median baseline body weight: 98.8 kg; median baseline body mass index: 35.4). The GLP-1/GLP-1/GIP group exhibited a higher incidence of AEs compared to the control group [OR 2.02 (95% CI 1.42 to 2.86)]. However, the number of serious AEs did not differ significantly between the GLP-1/GLP-1/GIP group and placebo groups [OR 1.01 (95% CI 0.83 to 1.22)]. Specifically, gastrointestinal side effects were notable in the GLP-1/GLP-1/GIP group. Nausea [OR 2.65 (95% CI 1.85 to 3.81, N=19)], vomiting [OR 3.51 (95% CI 2.21 to 5.59, N=19)], diarrhea [OR 2.55 (95% CI 2.08 to 3.12, N=18)], and cholecystitis [OR 2.12 (95% CI 1.26 to 3.58, N=15)] were significantly higher compared with placebo. Conversely, the rates of constipation [OR 1.52 (95% CI 0.96 to 2.42, N=18)], cholelithiasis [OR 1.31 (95% CI 0.89 to 1.92, N=15)], and pancreatitis [OR 0.83 (95% CI 0.48 to 1.41, N=9)] were comparable between the two groups.
Discussion:

Figure: Odds Ratios and 95% Confidence Intervals for Adverse Events and Gastrointestinal Side Effects with GLP-1 Receptor Agonists and GLP-1/GIP Coagonist in Chronic Weight Management.
Disclosures:
Lizeth Cifuentes indicated no relevant financial relationships.
Mehdi Abbasi indicated no relevant financial relationships.
Wissam Ghusn indicated no relevant financial relationships.
Domenica Jijon indicated no relevant financial relationships.
John Navarrete indicated no relevant financial relationships.
Mayte Insuasti indicated no relevant financial relationships.
Joseph Murphy indicated no relevant financial relationships.
Nanditha Venkatesan indicated no relevant financial relationships.
Shakira Yoosuf indicated no relevant financial relationships.
Anusha Amaravathi indicated no relevant financial relationships.
Mateo Fabara indicated no relevant financial relationships.
Lizeth Cifuentes, MD1, Mehdi Abbasi, MD2, Wissam Ghusn, MD3, Domenica Jijon, MD4, John Navarrete, MD4, Mayte Insuasti, MD4, Joseph M.. Murphy, MD4, Nanditha Venkatesan, MD1, Shakira Yoosuf, MD1, Anusha Amaravathi, MD1, Mateo Fabara, MD4. P4882 - Association of GLP-1 RA Or Dual GIP and GLP-1 RA for Obesity And Gastrointestinal Adverse Events: A Systematic Review, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.

