Tuesday Poster Session
Category: Practice Management
P4919 - Contemporary Landscape of Medical Technology in Gastroenterology Between 2013 and 2023: State-of-the-Art Scoping Review of the Clinical Evidence Base
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Megan Hennessey, MD
Dartmouth-Hitchcock Medical Center
Lebanon, NH
Presenting Author(s)
Megan Hennessey, MD1, Shan Xue, MD1, Rebecca Stringenz, MD1, Pamela Bagley, PhD2, Eric Shah, MD3
1Dartmouth-Hitchcock Medical Center, Lebanon, NH; 2Dartmouth College, Hanover, NH; 3University of Michigan, Ann Arbor, MI
Introduction: Gastroenterology is a technology-oriented field, yet gastroenterologists are unaware of the evidence to support the availability of devices they use daily. We performed the first scoping review to assess clinical evidence and trends in the gastroenterology medical technology development landscape over the past decade.
Methods: Our study was conducted according to the PRISMA-ScR checklist, and our research librarians rigorously developed our search strategy. Two reviewers independently assessed all medical technologies approved or cleared by the Food and Drug Administration (FDA) between 2013 and 2023 across FDA databases for 510(k) clearances, pre-market approvals (PMA), and De Novo classifications. Clinical trials used for regulatory evaluation were cross-linked using PubMed and EMBASE. Domains for this scoping review were defined on the area of intended use, availability of clinical trial data to support FDA approval/clearance, and (when available) adherence to CONSORT to assess study quality in med tech trials under the same lens as for drug trials.
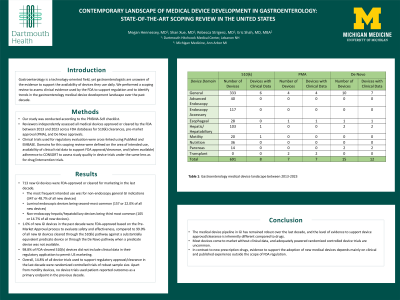
Results: 713 new GI technologies were FDA-approved or cleared for marketing in the last decade (TABLE). The most frequent intended use was for non-endoscopy general GI indications (347 or 48.7% of all new technologies), with luminal endoscopic devices being second-most common (157 or 22.0%) and non-endoscopy hepatic/hepatobiliary technologies being third most common (105 or 14.7%). 1.0% of all new GI technologies in the past decade were FDA-approved based on the Pre-Market Approval process to evaluate safety and effectiveness, compared to 99.0% of all new GI technologies in the past decade being FDA-cleared through the 510(k) pathway against a substantially equivalent predicate device or through the De Novo pathway when a predicate device was not available. 98.8% of FDA-cleared 510(k) technologies did not include clinical data in their regulatory application to permit US marketing. Overall, 14.8% of all GI medical technology trials used to support regulatory approval/clearance in the last decade were randomized controlled trials of robust sample size. Apart from motility technologies, no trials used patient-reported outcomes as a primary endpoint in the previous decade.
Discussion: The medical technology pipeline in gastroenterology is robust and broad in scope, supporting the necessary evolution that defines gastroenterology. Unlike drugs, evidence to support new medical technology depends mainly on clinical and published experience outside the scope of FDA regulation.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Megan Hennessey, MD1, Shan Xue, MD1, Rebecca Stringenz, MD1, Pamela Bagley, PhD2, Eric Shah, MD3. P4919 - Contemporary Landscape of Medical Technology in Gastroenterology Between 2013 and 2023: State-of-the-Art Scoping Review of the Clinical Evidence Base, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Dartmouth-Hitchcock Medical Center, Lebanon, NH; 2Dartmouth College, Hanover, NH; 3University of Michigan, Ann Arbor, MI
Introduction: Gastroenterology is a technology-oriented field, yet gastroenterologists are unaware of the evidence to support the availability of devices they use daily. We performed the first scoping review to assess clinical evidence and trends in the gastroenterology medical technology development landscape over the past decade.
Methods: Our study was conducted according to the PRISMA-ScR checklist, and our research librarians rigorously developed our search strategy. Two reviewers independently assessed all medical technologies approved or cleared by the Food and Drug Administration (FDA) between 2013 and 2023 across FDA databases for 510(k) clearances, pre-market approvals (PMA), and De Novo classifications. Clinical trials used for regulatory evaluation were cross-linked using PubMed and EMBASE. Domains for this scoping review were defined on the area of intended use, availability of clinical trial data to support FDA approval/clearance, and (when available) adherence to CONSORT to assess study quality in med tech trials under the same lens as for drug trials.
Results: 713 new GI technologies were FDA-approved or cleared for marketing in the last decade (TABLE). The most frequent intended use was for non-endoscopy general GI indications (347 or 48.7% of all new technologies), with luminal endoscopic devices being second-most common (157 or 22.0%) and non-endoscopy hepatic/hepatobiliary technologies being third most common (105 or 14.7%). 1.0% of all new GI technologies in the past decade were FDA-approved based on the Pre-Market Approval process to evaluate safety and effectiveness, compared to 99.0% of all new GI technologies in the past decade being FDA-cleared through the 510(k) pathway against a substantially equivalent predicate device or through the De Novo pathway when a predicate device was not available. 98.8% of FDA-cleared 510(k) technologies did not include clinical data in their regulatory application to permit US marketing. Overall, 14.8% of all GI medical technology trials used to support regulatory approval/clearance in the last decade were randomized controlled trials of robust sample size. Apart from motility technologies, no trials used patient-reported outcomes as a primary endpoint in the previous decade.
Discussion: The medical technology pipeline in gastroenterology is robust and broad in scope, supporting the necessary evolution that defines gastroenterology. Unlike drugs, evidence to support new medical technology depends mainly on clinical and published experience outside the scope of FDA regulation.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Megan Hennessey indicated no relevant financial relationships.
Shan Xue indicated no relevant financial relationships.
Rebecca Stringenz indicated no relevant financial relationships.
Pamela Bagley indicated no relevant financial relationships.
Eric Shah: Abbvie – Consultant. Ardelyx – Consultant. Cook – Consultant. Laborie – Consultant. Mahana – Consultant. Mylan – Consultant. Neuraxis – Consultant. Salix – Consultant. Sanofi – Consultant. Takeda – Consultant.
Megan Hennessey, MD1, Shan Xue, MD1, Rebecca Stringenz, MD1, Pamela Bagley, PhD2, Eric Shah, MD3. P4919 - Contemporary Landscape of Medical Technology in Gastroenterology Between 2013 and 2023: State-of-the-Art Scoping Review of the Clinical Evidence Base, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
