Tuesday Poster Session
Category: Small Intestine
P4935 - Efficacy and Safety of Latiglutinase In Celiac Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

- FJ
Fouad Jaber, MD, MS
University of Missouri - Kansas City School of Medicine
Kansas City, MO
Presenting Author(s)
Fouad Jaber, MD, MS1, Mohammed Ayyad, MD2, Saqr Alsakarneh, MD1, Mohammad Jaber, MD3, Anas Alselek, MD4, Mohammad Adam, MD5, Manesh Kumar Gangwani, MD6, Hassam Ali, MD7, Yazan Sallam, MD5, Raed Darwish, MD8, Ahmed Fares, MD9, Dushyant S. Dahiya, MD10
1University of Missouri - Kansas City School of Medicine, Kansas City, MO; 2Rutgers New Jersey Medical School, Newark, NJ; 3Al-Azhar University, Gaza, Palestinian Territories; 4Cairo University School of Medicine, Cairo, Al Jizah, Egypt; 5University of Missouri, Kansas City, MO; 6University of Toledo, Toledo, OH; 7ECU Health Medical Center, Greenville, NC; 8Ain Shams University, Cairo, Al Qahirah, Egypt; 9Tufts Medical Center, Boston, MA; 10The University of Kansas School of Medicine, Kansas City, KS
Introduction: Celiac disease is a chronic immune disorder triggered by dietary gluten, leading to mucosal inflammation in genetically predisposed individuals. Despite adherence to a gluten-free diet (GFD), many patients experience persistent symptoms and mucosal damage, prompting the exploration of non-dietary therapeutic approaches like latiglutenase. Latiglutenase is an enzyme therapy designed to degrade gluten proteins into non-immunogenic fragments, potentially reducing gut mucosa inflammation. This meta-analysis aimed to evaluate the efficacy and safety of latiglutenase in treating celiac disease.
Methods: A systematic review of major databases, including Cochrane, SCOPUS, PubMed, Web of Science, and EMBASE till April 2024 was conducted, adhering to PRISMA guidelines. Randomized controlled trials (RCTs) comparing latiglutenase to placebo in celiac disease patients were included. Risk ratios (RR) and mean differences (MD) with 95% confidence intervals (CI) were calculated using the DerSimonian–Laird method. Primary outcomes were the villus height to crypt depth ratio and intraepithelial lymphocyte densities; secondary outcomes included adverse events (AEs).
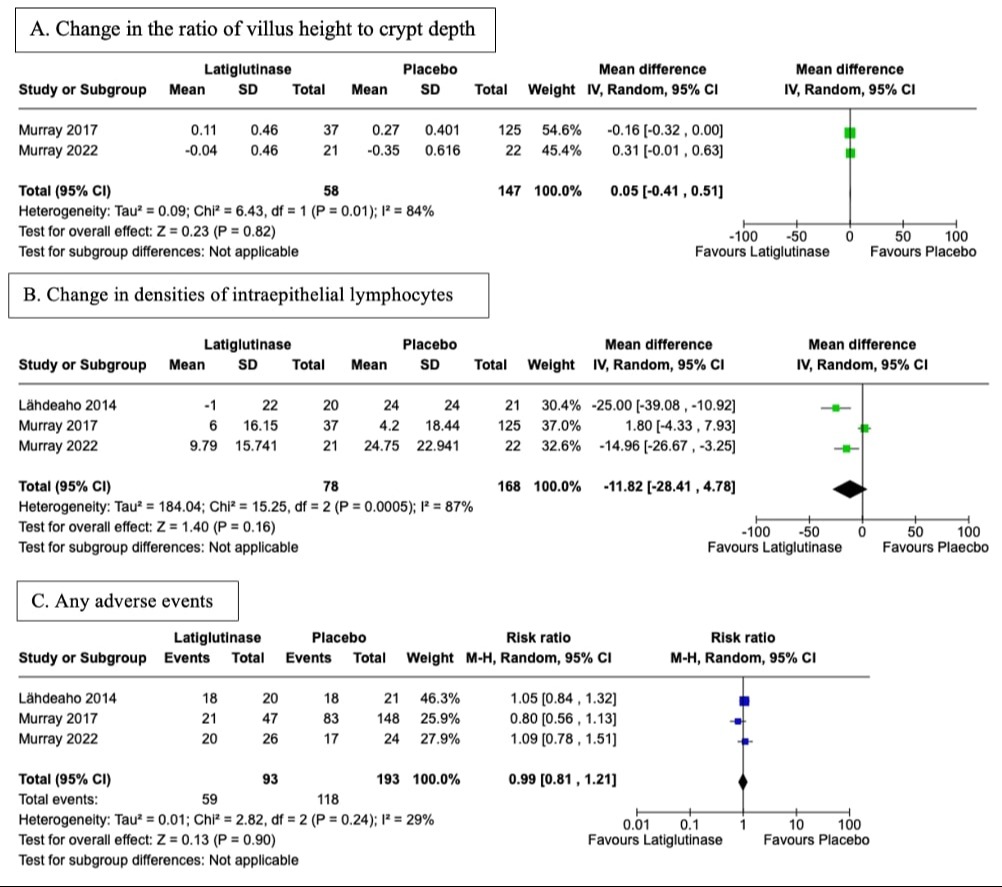
Results: Three RCTs involving 253 patients were analyzed (Table 1). No significant difference was observed between latiglutenase and placebo in the villus height to crypt depth ratio (MD: 0.05, 95% CI [-0.41, 0.51], P = 0.82) (Figure 1-A) or intraepithelial lymphocyte densities (MD: -11.82, 95% CI [-28.41, 4.78], P = 0.16) (Figure 1-B). Additionally, there were no significant differences in overall AEs (RR: 0.99, 95% CI [0.81, 1.21], P = 0.90), medication-related AEs (RR: 1.33, 95% CI [0.71, 2.49], P = 0.37), or gastrointestinal-related AEs (RR: 1.05, 95% CI [0.82, 1.34], P = 0.70) (Figure 1-C). Specific symptoms like diarrhea, nausea, headache, fatigue/tiredness, and abdominal distension did not differ between groups. Stratified by severity, both latiglutenase and placebo displayed comparable outcomes for mild, moderate, and severe AEs.
Discussion: While no significant differences were found in symptomatic, histological, and serological parameters between latiglutenase and placebo groups, previous trials indicated symptom improvements. This suggests that histological and serological parameters may require more time to normalize compared to symptomatic improvements. Longer-term RCTs are essential to comprehensively evaluate latiglutenase's effects on symptomatic, histological, and serological outcomes, along with its long-term safety profile.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Fouad Jaber, MD, MS1, Mohammed Ayyad, MD2, Saqr Alsakarneh, MD1, Mohammad Jaber, MD3, Anas Alselek, MD4, Mohammad Adam, MD5, Manesh Kumar Gangwani, MD6, Hassam Ali, MD7, Yazan Sallam, MD5, Raed Darwish, MD8, Ahmed Fares, MD9, Dushyant S. Dahiya, MD10. P4935 - Efficacy and Safety of Latiglutinase In Celiac Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Missouri - Kansas City School of Medicine, Kansas City, MO; 2Rutgers New Jersey Medical School, Newark, NJ; 3Al-Azhar University, Gaza, Palestinian Territories; 4Cairo University School of Medicine, Cairo, Al Jizah, Egypt; 5University of Missouri, Kansas City, MO; 6University of Toledo, Toledo, OH; 7ECU Health Medical Center, Greenville, NC; 8Ain Shams University, Cairo, Al Qahirah, Egypt; 9Tufts Medical Center, Boston, MA; 10The University of Kansas School of Medicine, Kansas City, KS
Introduction: Celiac disease is a chronic immune disorder triggered by dietary gluten, leading to mucosal inflammation in genetically predisposed individuals. Despite adherence to a gluten-free diet (GFD), many patients experience persistent symptoms and mucosal damage, prompting the exploration of non-dietary therapeutic approaches like latiglutenase. Latiglutenase is an enzyme therapy designed to degrade gluten proteins into non-immunogenic fragments, potentially reducing gut mucosa inflammation. This meta-analysis aimed to evaluate the efficacy and safety of latiglutenase in treating celiac disease.
Methods: A systematic review of major databases, including Cochrane, SCOPUS, PubMed, Web of Science, and EMBASE till April 2024 was conducted, adhering to PRISMA guidelines. Randomized controlled trials (RCTs) comparing latiglutenase to placebo in celiac disease patients were included. Risk ratios (RR) and mean differences (MD) with 95% confidence intervals (CI) were calculated using the DerSimonian–Laird method. Primary outcomes were the villus height to crypt depth ratio and intraepithelial lymphocyte densities; secondary outcomes included adverse events (AEs).
Results: Three RCTs involving 253 patients were analyzed (Table 1). No significant difference was observed between latiglutenase and placebo in the villus height to crypt depth ratio (MD: 0.05, 95% CI [-0.41, 0.51], P = 0.82) (Figure 1-A) or intraepithelial lymphocyte densities (MD: -11.82, 95% CI [-28.41, 4.78], P = 0.16) (Figure 1-B). Additionally, there were no significant differences in overall AEs (RR: 0.99, 95% CI [0.81, 1.21], P = 0.90), medication-related AEs (RR: 1.33, 95% CI [0.71, 2.49], P = 0.37), or gastrointestinal-related AEs (RR: 1.05, 95% CI [0.82, 1.34], P = 0.70) (Figure 1-C). Specific symptoms like diarrhea, nausea, headache, fatigue/tiredness, and abdominal distension did not differ between groups. Stratified by severity, both latiglutenase and placebo displayed comparable outcomes for mild, moderate, and severe AEs.
Discussion: While no significant differences were found in symptomatic, histological, and serological parameters between latiglutenase and placebo groups, previous trials indicated symptom improvements. This suggests that histological and serological parameters may require more time to normalize compared to symptomatic improvements. Longer-term RCTs are essential to comprehensively evaluate latiglutenase's effects on symptomatic, histological, and serological outcomes, along with its long-term safety profile.

Figure: Figure 1: Forest plot for A) Change in ratio of villus height to crypt ratio B) Change in densities of intraepithelial lymphocytes C) Any adverse events
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Fouad Jaber indicated no relevant financial relationships.
Mohammed Ayyad indicated no relevant financial relationships.
Saqr Alsakarneh indicated no relevant financial relationships.
Mohammad Jaber indicated no relevant financial relationships.
Anas Alselek indicated no relevant financial relationships.
Mohammad Adam indicated no relevant financial relationships.
Manesh Kumar Gangwani indicated no relevant financial relationships.
Hassam Ali indicated no relevant financial relationships.
Yazan Sallam indicated no relevant financial relationships.
Raed Darwish indicated no relevant financial relationships.
Ahmed Fares indicated no relevant financial relationships.
Dushyant Dahiya indicated no relevant financial relationships.
Fouad Jaber, MD, MS1, Mohammed Ayyad, MD2, Saqr Alsakarneh, MD1, Mohammad Jaber, MD3, Anas Alselek, MD4, Mohammad Adam, MD5, Manesh Kumar Gangwani, MD6, Hassam Ali, MD7, Yazan Sallam, MD5, Raed Darwish, MD8, Ahmed Fares, MD9, Dushyant S. Dahiya, MD10. P4935 - Efficacy and Safety of Latiglutinase In Celiac Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
