Tuesday Poster Session
Category: Small Intestine
P4969 - Acute Terminal Ileitis Secondary to FOLFOX Therapy for Stage IIIC Cecal Adenocarcinoma
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Amanda Jacubowsky, DO
Lehigh Valley Health Network
Allentown, PA
Presenting Author(s)
Amanda Jacubowsky, DO1, Tarika Chowdhary, MD1, Cristina Angelo, MD1, Tanveer Imam, MD2
1Lehigh Valley Health Network, Allentown, PA; 2Eastern Pennsylvania Gastrointestinal and Liver Specialists, Allentown, PA
Introduction: FOLFOX is a combination of 5-fluorouracil (5-FU), oxaliplatin, and leucovorin used to treat gastric, bowel, pancreatic, breast, and head and neck carcinomas. The common adverse effects include nausea, vomiting, moderate to severe diarrhea with colitis, stomatitis, and myelosuppression. Here we present a rare case of acute, severe ileitis with focal ulceration on colonoscopy secondary to FOLFOX therapy.
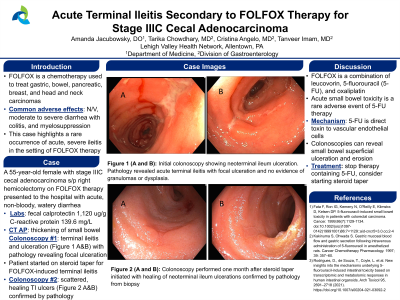
Case Description/Methods: A 55-year-old female with a past medical history of stage IIIC cecal adenocarcinoma s/p right hemicolectomy on FOLFOX therapy presented to the hospital with acute, non-bloody, watery diarrhea. She denied recent sick contacts and travel and noted she started FOLFOX 3 weeks prior. On admission, she was afebrile. Labs revealed a normal complete blood count, normal comprehensive metabolic panel, negative stool culture, fecal calprotectin 1,120 ug/g, and C-reactive protein 139.6 mg/L. CT abdomen and pelvis showed evidence of right hemicolectomy with thickening of small bowel extending to the anastomosis, suggesting active enteritis. Gastroenterology was consulted and a colonoscopy revealed terminal ileitis, rectal ulceration, and otherwise normal colonic mucosa. Biopsy results showed moderately active chronic colitis with acute, severe terminal ileitis with focal ulceration. There was no pathologic evidence of granulomas or dysplasia. A multidisciplinary discussion between gastroenterology, oncology, and pathology concluded the colonoscopy biopsy results were likely due to FOLFOX use and less likely secondary to inflammatory bowel disease. After shared decision-making, the patient elected to continue FOLFOX therapy for 6 months for her aggressive, poorly differentiated cecal adenocarcinoma and start a prolonged prednisone taper. A repeat colonoscopy 1 month after steroid taper was initiated showed normal anorectal and colonic mucosa with unremarkable biopsies.
Discussion: Acute small bowel toxicity from 5-FU therapy is a rare adverse event with only six reported cases to date. Patients typically present with abdominal pain, nausea, vomiting, and diarrhea. Colonoscopies can be performed to reveal small bowel superficial ulceration and erosion. The proposed mechanism of gastrointestinal injury is direct toxicity of 5-FU on vascular endothelial cells and inhibition of endothelium-derived relaxing factor secretion. The treatment includes decreasing the dose or discontinuation of FOLFOX. A steroid taper can also be used to decrease inflammation and reduce the frequency of diarrhea.
Disclosures:
Amanda Jacubowsky, DO1, Tarika Chowdhary, MD1, Cristina Angelo, MD1, Tanveer Imam, MD2. P4969 - Acute Terminal Ileitis Secondary to FOLFOX Therapy for Stage IIIC Cecal Adenocarcinoma, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Lehigh Valley Health Network, Allentown, PA; 2Eastern Pennsylvania Gastrointestinal and Liver Specialists, Allentown, PA
Introduction: FOLFOX is a combination of 5-fluorouracil (5-FU), oxaliplatin, and leucovorin used to treat gastric, bowel, pancreatic, breast, and head and neck carcinomas. The common adverse effects include nausea, vomiting, moderate to severe diarrhea with colitis, stomatitis, and myelosuppression. Here we present a rare case of acute, severe ileitis with focal ulceration on colonoscopy secondary to FOLFOX therapy.
Case Description/Methods: A 55-year-old female with a past medical history of stage IIIC cecal adenocarcinoma s/p right hemicolectomy on FOLFOX therapy presented to the hospital with acute, non-bloody, watery diarrhea. She denied recent sick contacts and travel and noted she started FOLFOX 3 weeks prior. On admission, she was afebrile. Labs revealed a normal complete blood count, normal comprehensive metabolic panel, negative stool culture, fecal calprotectin 1,120 ug/g, and C-reactive protein 139.6 mg/L. CT abdomen and pelvis showed evidence of right hemicolectomy with thickening of small bowel extending to the anastomosis, suggesting active enteritis. Gastroenterology was consulted and a colonoscopy revealed terminal ileitis, rectal ulceration, and otherwise normal colonic mucosa. Biopsy results showed moderately active chronic colitis with acute, severe terminal ileitis with focal ulceration. There was no pathologic evidence of granulomas or dysplasia. A multidisciplinary discussion between gastroenterology, oncology, and pathology concluded the colonoscopy biopsy results were likely due to FOLFOX use and less likely secondary to inflammatory bowel disease. After shared decision-making, the patient elected to continue FOLFOX therapy for 6 months for her aggressive, poorly differentiated cecal adenocarcinoma and start a prolonged prednisone taper. A repeat colonoscopy 1 month after steroid taper was initiated showed normal anorectal and colonic mucosa with unremarkable biopsies.
Discussion: Acute small bowel toxicity from 5-FU therapy is a rare adverse event with only six reported cases to date. Patients typically present with abdominal pain, nausea, vomiting, and diarrhea. Colonoscopies can be performed to reveal small bowel superficial ulceration and erosion. The proposed mechanism of gastrointestinal injury is direct toxicity of 5-FU on vascular endothelial cells and inhibition of endothelium-derived relaxing factor secretion. The treatment includes decreasing the dose or discontinuation of FOLFOX. A steroid taper can also be used to decrease inflammation and reduce the frequency of diarrhea.
Disclosures:
Amanda Jacubowsky indicated no relevant financial relationships.
Tarika Chowdhary indicated no relevant financial relationships.
Cristina Angelo indicated no relevant financial relationships.
Tanveer Imam indicated no relevant financial relationships.
Amanda Jacubowsky, DO1, Tarika Chowdhary, MD1, Cristina Angelo, MD1, Tanveer Imam, MD2. P4969 - Acute Terminal Ileitis Secondary to FOLFOX Therapy for Stage IIIC Cecal Adenocarcinoma, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
