Tuesday Poster Session
Category: Stomach
P5051 - Characteristics of Gastroparesis Clinical Studies Registered in Clinicaltrials.gov -- A Cross-Sectional Analysis
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- AT
Akanksha Togra, MD
Texas Tech University Health Sciences Center
El Paso, Texas
Presenting Author(s)
Akanksha Togra, MD1, M Ammar Kalas, MD1, Mohammad Bashashati, MD2, Irene Sarosiek, MD3, Richard W. McCallum, MD4
1Texas Tech University Health Sciences Center, El Paso, TX; 2University of Texas at Austin Dell Medical School, Austin, TX; 3Texas Tech University Health Sciences Center El Paso, El Paso, TX; 4Texas Tech University Health Sciences Center School of Medicine, El Paso, TX
Introduction: Gastroparesis (GP) has a high prevalence of around 9.6 to 37.8 per 100,000. Metoclopramide is the only FDA approved agent for GP making it a significant unmet medical need. As no drug has been approved since 1979, there is an urgent need for innovative treatment strategies. We analyzed trends in GP studies registered in ClinicalTrials.gov (CTG) to understand the ongoing research.
Methods: This is a cross-sectional study of GP studies registered in CTG from September 27, 2007 to April 30, 2024 identified using the ‘Advanced Search’ feature. Trial trends and characteristics were assessed through relative frequency calculations. Odds ratio and 95% confidence intervals were determined by comparing with total studies registered in CTG.
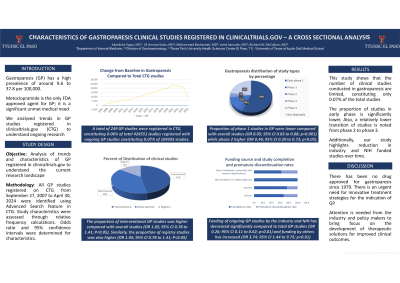
Results: A total of 249 GP studies were registered in CTG, constituting 0.06% of total 424551 studies registered with ongoing GP studies constituting 0.07% of 104592 studies.
Of 249 GP clinical studies, there were 192 (77.1%) interventional and 57 (22.9%) observational studies, with patient registries studies constituting 6.43%. The proportion of interventional GP studies was higher compared with overall studies (OR 1.05; 95 % CI: 0.78 to 1.41; P< 0.05). Similarly, the proportion of registry studies was also higher (OR 1.45; 95% CI: 1.41 to 4.28; P< 0.01).
A total of 68 (27.3%) GP studies were sponsored by industry; 21 (8.4%) by NIH and other US Federal agencies; and 192 studies (77.1%) by all others (individuals, universities, research organizations). These funding trends are similar to overall studies registered. To understand trend over time, we compared funding of ongoing GP studies with total GP studies registered and found that funding by the industry and NIH has decreased significantly (OR 0.26; 95% CI 0.11 to 0.62; P< 0.01) and funding by others has increased (OR 3.74; 95% CI: 1.44 to 9.75; P< 0.01).
The proportion of phase 1 studies in GP were lower (OR 0.05; 95 % CI: 0.03 to 0.08; P< 0.001) and the proportion of phase 2 studies is higher (OR 1.79; 95 % CI: 1.34 to 2.41; P< 0.001) compared to overall studies registered. Proportion of phase 3 studies was not statistically different.
Discussion: This study shows that the number of clinical studies conducted in GP are limited. Our study indicates drop in industry funded studies over time and relatively lower transition from phase 2 to phase 3. Attention is needed from the industry and policy makers to bring focus on the development of therapeutic solutions for improved clinical outcomes.
Disclosures:
Akanksha Togra, MD1, M Ammar Kalas, MD1, Mohammad Bashashati, MD2, Irene Sarosiek, MD3, Richard W. McCallum, MD4. P5051 - Characteristics of Gastroparesis Clinical Studies Registered in Clinicaltrials.gov -- A Cross-Sectional Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Texas Tech University Health Sciences Center, El Paso, TX; 2University of Texas at Austin Dell Medical School, Austin, TX; 3Texas Tech University Health Sciences Center El Paso, El Paso, TX; 4Texas Tech University Health Sciences Center School of Medicine, El Paso, TX
Introduction: Gastroparesis (GP) has a high prevalence of around 9.6 to 37.8 per 100,000. Metoclopramide is the only FDA approved agent for GP making it a significant unmet medical need. As no drug has been approved since 1979, there is an urgent need for innovative treatment strategies. We analyzed trends in GP studies registered in ClinicalTrials.gov (CTG) to understand the ongoing research.
Methods: This is a cross-sectional study of GP studies registered in CTG from September 27, 2007 to April 30, 2024 identified using the ‘Advanced Search’ feature. Trial trends and characteristics were assessed through relative frequency calculations. Odds ratio and 95% confidence intervals were determined by comparing with total studies registered in CTG.
Results: A total of 249 GP studies were registered in CTG, constituting 0.06% of total 424551 studies registered with ongoing GP studies constituting 0.07% of 104592 studies.
Of 249 GP clinical studies, there were 192 (77.1%) interventional and 57 (22.9%) observational studies, with patient registries studies constituting 6.43%. The proportion of interventional GP studies was higher compared with overall studies (OR 1.05; 95 % CI: 0.78 to 1.41; P< 0.05). Similarly, the proportion of registry studies was also higher (OR 1.45; 95% CI: 1.41 to 4.28; P< 0.01).
A total of 68 (27.3%) GP studies were sponsored by industry; 21 (8.4%) by NIH and other US Federal agencies; and 192 studies (77.1%) by all others (individuals, universities, research organizations). These funding trends are similar to overall studies registered. To understand trend over time, we compared funding of ongoing GP studies with total GP studies registered and found that funding by the industry and NIH has decreased significantly (OR 0.26; 95% CI 0.11 to 0.62; P< 0.01) and funding by others has increased (OR 3.74; 95% CI: 1.44 to 9.75; P< 0.01).
The proportion of phase 1 studies in GP were lower (OR 0.05; 95 % CI: 0.03 to 0.08; P< 0.001) and the proportion of phase 2 studies is higher (OR 1.79; 95 % CI: 1.34 to 2.41; P< 0.001) compared to overall studies registered. Proportion of phase 3 studies was not statistically different.
Discussion: This study shows that the number of clinical studies conducted in GP are limited. Our study indicates drop in industry funded studies over time and relatively lower transition from phase 2 to phase 3. Attention is needed from the industry and policy makers to bring focus on the development of therapeutic solutions for improved clinical outcomes.
Disclosures:
Akanksha Togra: Clinexel Inc – Owner/Ownership Interest. Clinexel Life Sciences Pvt Ltd – Owner/Ownership Interest. Cytenet Life science LLP – Owner/Ownership Interest. GLRK Healthcare foundation (Non-profit Organization Company) – Owner/Ownership Interest.
M Ammar Kalas indicated no relevant financial relationships.
Mohammad Bashashati indicated no relevant financial relationships.
Irene Sarosiek indicated no relevant financial relationships.
Richard McCallum: Evoke Pharma – Consultant.
Akanksha Togra, MD1, M Ammar Kalas, MD1, Mohammad Bashashati, MD2, Irene Sarosiek, MD3, Richard W. McCallum, MD4. P5051 - Characteristics of Gastroparesis Clinical Studies Registered in Clinicaltrials.gov -- A Cross-Sectional Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
