Tuesday Poster Session
Category: Interventional Endoscopy
P4513 - Initial Experience With the Transmural Use of a New Endoscopic Ultrasound Core Needle Biopsy Device: A Case Series
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Omar Viramontes, MD
University of California Davis Health
Sacramento, CA
Presenting Author(s)
Omar Viramontes, MD, Amer Alsamman, MD, Karleen M. Meiklejohn, MD, Michael Larson, MD, Antonio Mendoza Ladd, MD
University of California Davis Health, Sacramento, CA
Introduction: Endoscopic Ultrasound Guided Tissue Acquisition (EUSTGA) via Fine Needle Aspiration (FNA) and Biopsy (FNB) is currently the method of choice to sample tumors in the mediastinum and abdomen. Although these needles are effective, a histological diagnosis is not possible in a significant percentage of cases due to scarce tissue quantity and fragmented tissue cores. Recently, a new EUS-guided core needle biopsy (EUS-CNB) device became available. The only available literature on this device is on sampling of submucosal lesions. In this series we describe the first clinical experience using it in a transmural fashion.
Case Description/Methods: This was a case series of hospitalized patients who underwent EUS-CNB of a variety of tumors from January-March of 2024 at a single academic center. Demographic and clinical information was obtained from the medical record. The focus of it was to evaluate the diagnostic performance and safety profile of a new core needle (17G) biopsy device. This new device is an electromechanical device that allows procurement of EUS-CNB. This new EUS-CNB device consists of a hollow flexible rotating cylinder that is covered by a flexible metal sheath. Rotation is facilitated by a stand-alone motor connected to the handle with a drive cable and controlled with a pedal.
A total of 5 patients underwent EUS-CNB: 4 for pancreatic tumors and 1 for a retroperitoneal mass (table 1). The diagnostic accuracy of EUS-CNB was 100% after one pass and all specimens were submitted as surgical specimens. In two patients, both FNB and EUS-CNB were obtained from the same lesion during the same procedure, with superior tissue samples in the latter. No adverse events were documented.
Discussion: To our knowledge, this is the first report on the transmural use of EUS-CNB in gastroenterology. In this initial series the device was effective, and safe. Larger prospective studies are needed comparing this device to standard FNA and/or FNB needles.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Omar Viramontes, MD, Amer Alsamman, MD, Karleen M. Meiklejohn, MD, Michael Larson, MD, Antonio Mendoza Ladd, MD. P4513 - Initial Experience With the Transmural Use of a New Endoscopic Ultrasound Core Needle Biopsy Device: A Case Series, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
University of California Davis Health, Sacramento, CA
Introduction: Endoscopic Ultrasound Guided Tissue Acquisition (EUSTGA) via Fine Needle Aspiration (FNA) and Biopsy (FNB) is currently the method of choice to sample tumors in the mediastinum and abdomen. Although these needles are effective, a histological diagnosis is not possible in a significant percentage of cases due to scarce tissue quantity and fragmented tissue cores. Recently, a new EUS-guided core needle biopsy (EUS-CNB) device became available. The only available literature on this device is on sampling of submucosal lesions. In this series we describe the first clinical experience using it in a transmural fashion.
Case Description/Methods: This was a case series of hospitalized patients who underwent EUS-CNB of a variety of tumors from January-March of 2024 at a single academic center. Demographic and clinical information was obtained from the medical record. The focus of it was to evaluate the diagnostic performance and safety profile of a new core needle (17G) biopsy device. This new device is an electromechanical device that allows procurement of EUS-CNB. This new EUS-CNB device consists of a hollow flexible rotating cylinder that is covered by a flexible metal sheath. Rotation is facilitated by a stand-alone motor connected to the handle with a drive cable and controlled with a pedal.
A total of 5 patients underwent EUS-CNB: 4 for pancreatic tumors and 1 for a retroperitoneal mass (table 1). The diagnostic accuracy of EUS-CNB was 100% after one pass and all specimens were submitted as surgical specimens. In two patients, both FNB and EUS-CNB were obtained from the same lesion during the same procedure, with superior tissue samples in the latter. No adverse events were documented.
Discussion: To our knowledge, this is the first report on the transmural use of EUS-CNB in gastroenterology. In this initial series the device was effective, and safe. Larger prospective studies are needed comparing this device to standard FNA and/or FNB needles.

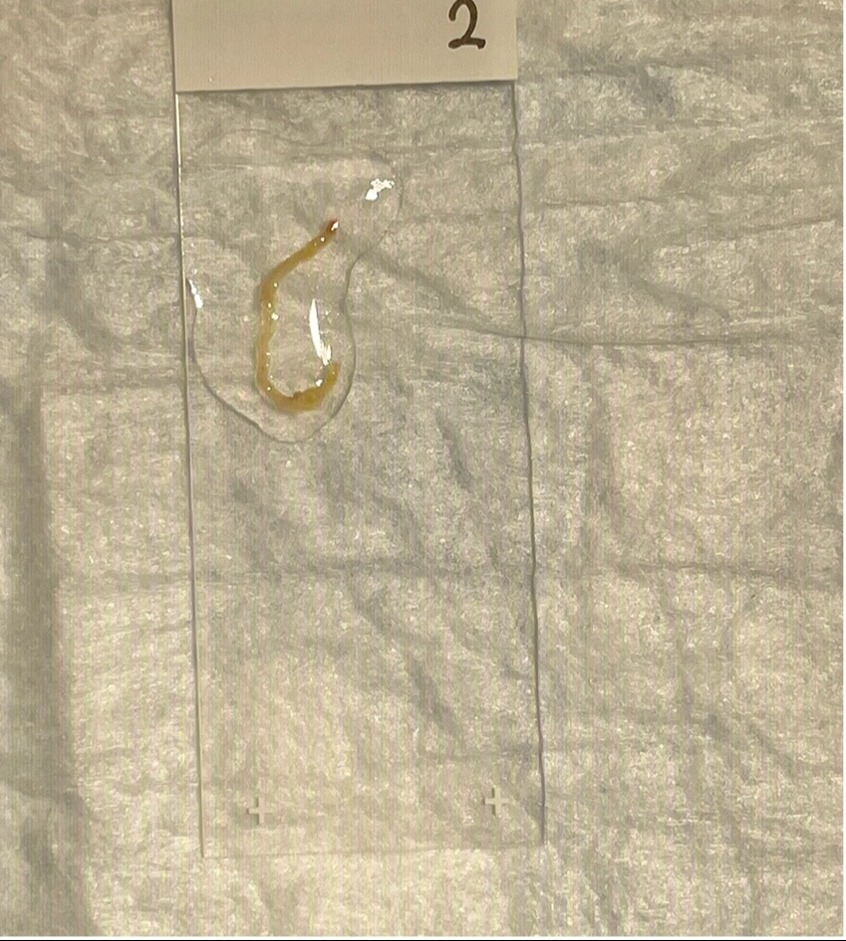
Figure: Figure 1: Core Needle Biopsy (CNB)
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Omar Viramontes indicated no relevant financial relationships.
Amer Alsamman indicated no relevant financial relationships.
Karleen Meiklejohn indicated no relevant financial relationships.
Michael Larson indicated no relevant financial relationships.
Antonio Mendoza Ladd: Boston Scientific – Consultant. Nestle – Speakers Bureau. Olympus – Consultant.
Omar Viramontes, MD, Amer Alsamman, MD, Karleen M. Meiklejohn, MD, Michael Larson, MD, Antonio Mendoza Ladd, MD. P4513 - Initial Experience With the Transmural Use of a New Endoscopic Ultrasound Core Needle Biopsy Device: A Case Series, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
