Tuesday Poster Session
Category: IBD
P4353 - Impact of Prior Biologic Exposure on the Durability of Ozanimod Over 5 Years of Continuous Treatment in Patients With Ulcerative Colitis: An Interim Analysis of the True North Open-Label Extension Study
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Bruce E. Sands, MD, FACG
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Bruce E.. Sands, MD, FACG1, Caterina Oneto, MD2, Zachary A. Borman, MD3, Gaurav Syal, MD, MHDS4, Ryan C. Ungaro, MD1, Marc Ferrante, MD, PhD5, James O. Lindsay, PhD6, Irina Blumenstein, MD, PhD7, Zhaohui Liu, PhD8, Dimpy Mehra, PharmD8, Anjali Jain, PhD8, Geert R. D'Haens, MD, PhD9
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Vanguard Gastroenterology, New York, NY; 3Capital Digestive Care, North Bethesda, MD; 4UC San Diego Health, La Jolla, CA; 5University Hospitals, Leuven, Vlaams-Brabant, Belgium; 6Barts and the London School of Medicine and Dentistry, London, England, United Kingdom; 7Goethe University Hospital, Frankfurt, Hamburg, Germany; 8Bristol Myers Squibb, Princeton, NJ; 9Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands
Introduction: Ozanimod (OZA) is approved for the treatment of moderately to severely active ulcerative colitis (UC) based on results from the phase 3 True North (TN) study (NCT02435992). OZA efficacy was durable for up to ~5 years of continuous treatment (Week [W] 190 in the TN open-label extension [OLE]; NCT02531126). This interim analysis of the TN OLE evaluated the effect of prior biologic (bio) exposure on the durability of OZA efficacy.
Methods: OZA clinical responders at TN W52 who entered the OLE were analyzed (cutoff: January 10, 2024). The trajectories of total and partial Mayo scores were assessed during TN and the OLE. Efficacy outcomes were evaluated through OLE W190 using observed case (OC) and nonresponder imputation (NRI) analyses. Data were evaluated by prior bio exposure at TN baseline. Patients (pts) with prior exposure to Janus kinase inhibitors only were excluded.
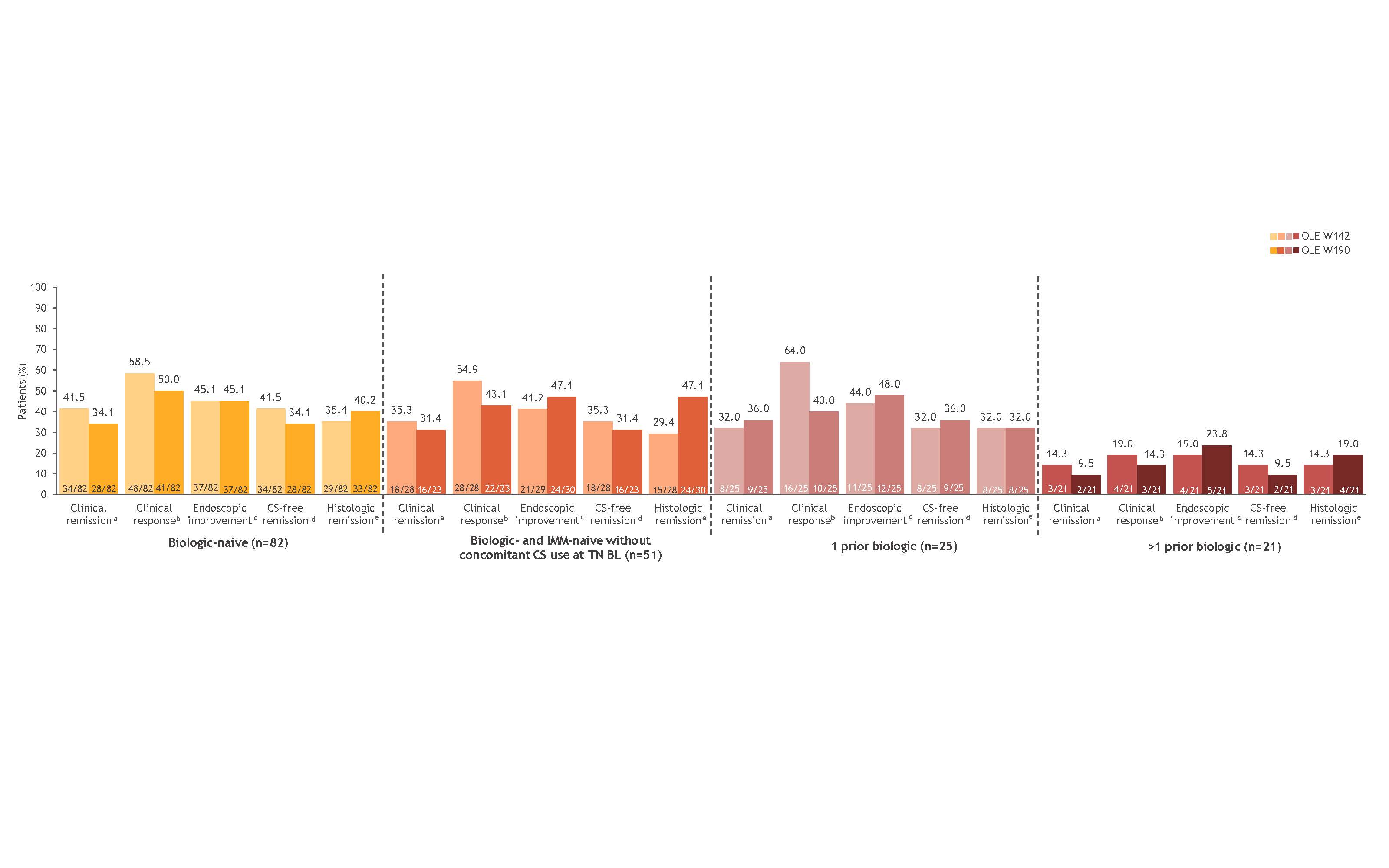
Results: Of the 128 TN W52 clinical responders who entered the OLE and had information on prior bio exposure, 82 were bio-naive (51 were bio-naive and immunomodulator [IMM]-naive without concomitant corticosteroid [CS] use at TN baseline), 25 were exposed to 1 prior bio, and 21 were exposed to >1 prior bio. Baseline pt characteristics were generally similar among groups, except pts with prior bio exposure had longer disease duration and higher prior CS and IMM use than the bio-naive pts. Mean total and partial Mayo scores were high at TN baseline but reduced by OLE entry in all groups, regardless of prior bio exposure; mean scores were generally maintained and similar among groups at OLE W190 (Table). Symptomatic efficacy rates were generally maintained in most pts through OLE W190 regardless of prior bio exposure. Clinical and mucosal efficacy rates were maintained through OLE W190, regardless of prior bio exposure (NRI analysis) (Figure). Pts who were bio-naive generally had the highest efficacy rates; however, rates were comparable to those in pts exposed to 1 prior bio. Although OZA efficacy was durable in >1 prior bio-exposed pts, efficacy rates were lower compared with bio-naive pts and pts exposed to 1 prior bio. Similar overall trends were observed in the OC analysis.
Discussion: OZA efficacy was durable for up to ~5 years, regardless of prior bio exposure, in pts with moderately to severely active UC. Long-term OZA efficacy was comparable in bio-naive pts and in pts exposed to 1 prior bio.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Bruce E.. Sands, MD, FACG1, Caterina Oneto, MD2, Zachary A. Borman, MD3, Gaurav Syal, MD, MHDS4, Ryan C. Ungaro, MD1, Marc Ferrante, MD, PhD5, James O. Lindsay, PhD6, Irina Blumenstein, MD, PhD7, Zhaohui Liu, PhD8, Dimpy Mehra, PharmD8, Anjali Jain, PhD8, Geert R. D'Haens, MD, PhD9. P4353 - Impact of Prior Biologic Exposure on the Durability of Ozanimod Over 5 Years of Continuous Treatment in Patients With Ulcerative Colitis: An Interim Analysis of the True North Open-Label Extension Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Vanguard Gastroenterology, New York, NY; 3Capital Digestive Care, North Bethesda, MD; 4UC San Diego Health, La Jolla, CA; 5University Hospitals, Leuven, Vlaams-Brabant, Belgium; 6Barts and the London School of Medicine and Dentistry, London, England, United Kingdom; 7Goethe University Hospital, Frankfurt, Hamburg, Germany; 8Bristol Myers Squibb, Princeton, NJ; 9Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands
Introduction: Ozanimod (OZA) is approved for the treatment of moderately to severely active ulcerative colitis (UC) based on results from the phase 3 True North (TN) study (NCT02435992). OZA efficacy was durable for up to ~5 years of continuous treatment (Week [W] 190 in the TN open-label extension [OLE]; NCT02531126). This interim analysis of the TN OLE evaluated the effect of prior biologic (bio) exposure on the durability of OZA efficacy.
Methods: OZA clinical responders at TN W52 who entered the OLE were analyzed (cutoff: January 10, 2024). The trajectories of total and partial Mayo scores were assessed during TN and the OLE. Efficacy outcomes were evaluated through OLE W190 using observed case (OC) and nonresponder imputation (NRI) analyses. Data were evaluated by prior bio exposure at TN baseline. Patients (pts) with prior exposure to Janus kinase inhibitors only were excluded.
Results: Of the 128 TN W52 clinical responders who entered the OLE and had information on prior bio exposure, 82 were bio-naive (51 were bio-naive and immunomodulator [IMM]-naive without concomitant corticosteroid [CS] use at TN baseline), 25 were exposed to 1 prior bio, and 21 were exposed to >1 prior bio. Baseline pt characteristics were generally similar among groups, except pts with prior bio exposure had longer disease duration and higher prior CS and IMM use than the bio-naive pts. Mean total and partial Mayo scores were high at TN baseline but reduced by OLE entry in all groups, regardless of prior bio exposure; mean scores were generally maintained and similar among groups at OLE W190 (Table). Symptomatic efficacy rates were generally maintained in most pts through OLE W190 regardless of prior bio exposure. Clinical and mucosal efficacy rates were maintained through OLE W190, regardless of prior bio exposure (NRI analysis) (Figure). Pts who were bio-naive generally had the highest efficacy rates; however, rates were comparable to those in pts exposed to 1 prior bio. Although OZA efficacy was durable in >1 prior bio-exposed pts, efficacy rates were lower compared with bio-naive pts and pts exposed to 1 prior bio. Similar overall trends were observed in the OC analysis.
Discussion: OZA efficacy was durable for up to ~5 years, regardless of prior bio exposure, in pts with moderately to severely active UC. Long-term OZA efficacy was comparable in bio-naive pts and in pts exposed to 1 prior bio.

Figure: Figure. Ozanimod efficacy at OLE W142 and OLE W190 by prior biologic exposure at BL in TN W52 clinical responders who entered the OLE (NRI analysis). Denominators for the NRI analysis were based on the numbers of patients who completed the OLE timepoint or discontinued ozanimod treatment. aRBS = 0, SFS ≤1 (and ≥1-point decrease from BL SFS), and MES ≤1. b≥2-point and ≥35% decrease from BL in the 3-component Mayo score (sum of RBS, SFS, and MES) and ≥1-point decrease in RBS or absolute RBS of ≤1. cMES ≤1. dClinical remission while off CS for ≥12 weeks. eGeboes score <2.0. BL, baseline; CS, corticosteroid; IMM, immunomodulator; MES, Mayo endoscopy subscore; NRI, nonresponder imputation; OLE, open-label extension; RBS, rectal bleeding subscore; SFS, stool frequency subscore; TN, True North; W, Week.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Agomab – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Other support, Speakers Bureau. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Enthera – Consultant. Envied Biosciences – Consultant. Equilium – Consultant. Evommune – Consultant. Ferring – Consultant. Fiat – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. Glaxo SmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen – Consultant, Grant/Research Support, Other support, Speakers Bureau. Kaleido – Consultant. Kallyope – Consultant. Lilly – Consultant, other support, Speakers Bureau. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, Other support, Speakers Bureau. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, Other support, Speakers Bureau. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biopharma – Consultant, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Caterina Oneto: AbbVie – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Phathom – Consultant, Speakers Bureau. Salix – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau.
Zachary A. Borman: AbbVie – Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Phathom – Speakers Bureau. Sanofi – Advisory Committee/Board Member, Consultant. Takeda – Speakers Bureau.
Gaurav Syal: Pfizer – Grant/Research Support.
Ryan C. Ungaro: AbbVie – Advisory Committee/Board Member, Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member, Consultant. Eli Lilly – Grant/Research Support. Inotrem – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Grant/Research Support. Prometheus Labratories – Grant/Research Support. Roivant – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member.
Marc Ferrante: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Agomab – Consultant. Amgen – Grant/Research Support, Speakers Bureau. Biogen – Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Celgene – Consultant. Celltrion – Consultant. Dr Falk Pharma – Speakers Bureau. EG Pharmaceuticals – Grant/Research Support. Eli Lilly and Company – Consultant, Grant/Research Support. Ferring – Speakers Bureau. Janssen – Grant/Research Support. Janssen-Cilag – Consultant, Speakers Bureau. Lamepro – Speakers Bureau. Medtronic – Consultant. MRM Health – Consultant. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Regeneron – Consultant. Samsung Bioepis – Consultant. Sandoz – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. ThermoFisher – Consultant. Truvion Healthcare – Speakers Bureau. Viatris – Grant/Research Support, Speakers Bureau.
James O. Lindsay: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Celgene – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Engitix – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Gilead – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Napp – Consultant, Speakers Bureau. Orchard Therapeutics – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau.
Irina Blumenstein: AbbVie – Consultant, Speakers Bureau. Amgen – Consultant, Speakers Bureau. Arena – Consultant, Speakers Bureau. Biogen – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Celgene – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Mylan – Speakers Bureau. Pfizer – Consultant, Stock Options. Shire – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau.
Zhaohui Liu: Bristol Myers Squibb – Employee.
Dimpy Mehra: Bristol Myers Squibb – Employee.
Anjali Jain: Bristol Myers Squibb – Employee.
Geert D'Haens: AbbVie – Advisor or Review Panel Member, Speakers Bureau. Agomab Therapeutics – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. Allergan – Advisor or Review Panel Member. Alphabiomics – Advisor or Review Panel Member. AstraZeneca – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Speakers Bureau. Ferring – Advisor or Review Panel Member. Galapagos – Advisor or Review Panel Member, Speakers Bureau. GlaxoSmithKline – Advisor or Review Panel Member. Immunic – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Seres – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau. Tillotts – Advisor or Review Panel Member, Speakers Bureau. Ventyx – Advisor or Review Panel Member.
Bruce E.. Sands, MD, FACG1, Caterina Oneto, MD2, Zachary A. Borman, MD3, Gaurav Syal, MD, MHDS4, Ryan C. Ungaro, MD1, Marc Ferrante, MD, PhD5, James O. Lindsay, PhD6, Irina Blumenstein, MD, PhD7, Zhaohui Liu, PhD8, Dimpy Mehra, PharmD8, Anjali Jain, PhD8, Geert R. D'Haens, MD, PhD9. P4353 - Impact of Prior Biologic Exposure on the Durability of Ozanimod Over 5 Years of Continuous Treatment in Patients With Ulcerative Colitis: An Interim Analysis of the True North Open-Label Extension Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
