Tuesday Poster Session
Category: IBD
P4263 - Black Patients with Inflammatory Bowel Disease are Less Likely to Reach Therapeutic Titers of Adalimumab Upon Initial Testing
Tuesday, October 29, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio

Landen Shane Burstiner, DO, MSc
University of Florida College of Medicine
Jacksonville, FL
Presenting Author(s)
Landen Shane Burstiner, DO, MSc1, Tomas Potlach, BS2, Nadim A. Qadir, DO3, Gerardo Diaz Garcia, DO1, Anvit D. Reddy, DO1, Tara Kronen, DO, MA1, Luke Stachler, MD1, Maged A.. Ghali, MD1, Ellen M. Zimmermann, MD4, Naueen A. Chaudhry, MD, MS4
1University of Florida College of Medicine, Jacksonville, FL; 2University of Florida College of Medicine, Miami, FL; 3University of Florida College of Medicine, Windermere, FL; 4University of Florida, Gainesville, FL
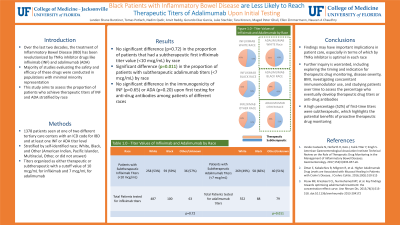
Introduction: Over the last two decades, the treatment of Inflammatory Bowel Disease (IBD) has been revolutionized by TNFα inhibitor drugs like infliximab (INF) and adalimumab (ADA). The majority of studies evaluating the safety and efficacy of these drugs were conducted in populations with minimal, or zero non-white patients. This study aims to assess the proportion of patients who achieve therapeutic titers of INF and ADA stratified by race.
Methods: Our study included all patients seen at one of two different tertiary care centers with an ICD9 or ICD10 code for IBD and at least one INF or ADA titer level. Overall, 1378 patients met inclusion criteria and 9 were excluded due to laboratory malfunctions or improper samples. Patients who self-identified as American Indian, Pacific Islander, Multiracial, Other, or did not answer were summarized in the “Other/Unknown” category.
Results: There was not a significant difference (p=0.72) in the proportion of patients that had a subtherapeutic first INF titer value (< 10 mcg/mL) among black patients, white patients, and patients of other/unknown race, as seen in Table 1. In contrast, there was a significant difference (p=0.011) in the proportion of patients with subtherapeutic ADA titers (< 7 mcg/mL). There was not a significant difference in the immunogenicity of INF (p=0.65) or ADA (p=0.20) upon first testing for anti-drug antibodies among white, black, and other patients with IBD.
Discussion: Our finding that black patients with IBD were similarly likely to reach therapeutic titers on INF, but less likely to do so with ADA, as compared to white patients, could have important implications in patient care. Further inquiry is warranted, including exploring the timing and indication for therapeutic drug monitoring, disease severity, BMI, investigating concomitant immunomodulator use, and studying patients over time to assess the percentage who eventually develop therapeutic drug titers or anti-drug antibodies. A high percentage (52%) of first-time titers were subtherapeutic, which highlights the potential benefits of proactive therapeutic drug monitoring.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Landen Shane Burstiner, DO, MSc1, Tomas Potlach, BS2, Nadim A. Qadir, DO3, Gerardo Diaz Garcia, DO1, Anvit D. Reddy, DO1, Tara Kronen, DO, MA1, Luke Stachler, MD1, Maged A.. Ghali, MD1, Ellen M. Zimmermann, MD4, Naueen A. Chaudhry, MD, MS4. P4263 - Black Patients with Inflammatory Bowel Disease are Less Likely to Reach Therapeutic Titers of Adalimumab Upon Initial Testing, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Florida College of Medicine, Jacksonville, FL; 2University of Florida College of Medicine, Miami, FL; 3University of Florida College of Medicine, Windermere, FL; 4University of Florida, Gainesville, FL
Introduction: Over the last two decades, the treatment of Inflammatory Bowel Disease (IBD) has been revolutionized by TNFα inhibitor drugs like infliximab (INF) and adalimumab (ADA). The majority of studies evaluating the safety and efficacy of these drugs were conducted in populations with minimal, or zero non-white patients. This study aims to assess the proportion of patients who achieve therapeutic titers of INF and ADA stratified by race.
Methods: Our study included all patients seen at one of two different tertiary care centers with an ICD9 or ICD10 code for IBD and at least one INF or ADA titer level. Overall, 1378 patients met inclusion criteria and 9 were excluded due to laboratory malfunctions or improper samples. Patients who self-identified as American Indian, Pacific Islander, Multiracial, Other, or did not answer were summarized in the “Other/Unknown” category.
Results: There was not a significant difference (p=0.72) in the proportion of patients that had a subtherapeutic first INF titer value (< 10 mcg/mL) among black patients, white patients, and patients of other/unknown race, as seen in Table 1. In contrast, there was a significant difference (p=0.011) in the proportion of patients with subtherapeutic ADA titers (< 7 mcg/mL). There was not a significant difference in the immunogenicity of INF (p=0.65) or ADA (p=0.20) upon first testing for anti-drug antibodies among white, black, and other patients with IBD.
Discussion: Our finding that black patients with IBD were similarly likely to reach therapeutic titers on INF, but less likely to do so with ADA, as compared to white patients, could have important implications in patient care. Further inquiry is warranted, including exploring the timing and indication for therapeutic drug monitoring, disease severity, BMI, investigating concomitant immunomodulator use, and studying patients over time to assess the percentage who eventually develop therapeutic drug titers or anti-drug antibodies. A high percentage (52%) of first-time titers were subtherapeutic, which highlights the potential benefits of proactive therapeutic drug monitoring.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Landen Shane Burstiner indicated no relevant financial relationships.
Tomas Potlach indicated no relevant financial relationships.
Nadim Qadir indicated no relevant financial relationships.
Gerardo Diaz Garcia indicated no relevant financial relationships.
Anvit Reddy indicated no relevant financial relationships.
Tara Kronen indicated no relevant financial relationships.
Luke Stachler indicated no relevant financial relationships.
Maged Ghali: Gilead – Grant/Research Support. Madrigal – Grant/Research Support. Novo Nordisk – Grant/Research Support.
Ellen Zimmermann indicated no relevant financial relationships.
Naueen Chaudhry indicated no relevant financial relationships.
Landen Shane Burstiner, DO, MSc1, Tomas Potlach, BS2, Nadim A. Qadir, DO3, Gerardo Diaz Garcia, DO1, Anvit D. Reddy, DO1, Tara Kronen, DO, MA1, Luke Stachler, MD1, Maged A.. Ghali, MD1, Ellen M. Zimmermann, MD4, Naueen A. Chaudhry, MD, MS4. P4263 - Black Patients with Inflammatory Bowel Disease are Less Likely to Reach Therapeutic Titers of Adalimumab Upon Initial Testing, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
