Monday Poster Session
Category: IBD
P2616 - Pneumococcal Vaccine is Associated with Improved Outcomes in Inflammatory Bowel Disease: Insights From a Propensity-Matched Study in the United States
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Has Audio

Mouhand F.H Mohamed, MD, MSc
Mayo Clinic
Rochester, MN
Presenting Author(s)
Mouhand F.H. Mohamed, MD, MSc1, Azizullah Beran, MD2, Osama Hamid, MD3, Francis A. Farraye, MD, MSc4, Samir A. Shah, MD5
1Mayo Clinic, Rochester, MN; 2Indiana University School of Medicine, Indianapolis, IN; 3University of Texas Southwestern Medical Center, Dallas, TX; 4Mayo Clinic, Jacksonville, FL; 5Gastroenterology Associates, Inc., powered by GI Alliance, Providence, RI
Introduction: The pneumococcal vaccine is recommended for patients with inflammatory bowel disease (IBD) due to an increased risk of pneumonia. The aim of this study was to evaluate pneumonia related outcomes in patients with IBD who received pneumococcal vaccination.
Methods: We analyzed TriNetX US Collaborative Network data, including > 100 million patient records from 64 healthcare organizations. Patients with IBD age >18 were included (January 2021 to June 2024). Using ICD-10 codes, we included Ulcerative colitis (UC) or Crohn's disease (CD) with at least one IBD therapy. Due to its single-dose regimen, we only included the pneumococcal vaccine 20-valent (PCV20) in the study as a surrogate for complete pneumococcal vaccination. For the control group, we excluded all forms of pneumococcal vaccines. A propensity score matching (PSM) accounting for demographics and pneumonia risk factors. We assessed outcomes > 30 days after vaccine administration. The primary outcome was the pneumonia risk. Other outcomes included hospitalizations, intensive care unit (ICU) services, acute respiratory failure (ARF), and all-cause mortality. We generated relative risk (RR) with 95% confidence intervals (CI). We performed a sub-group analysis for the primary outcome in patients < 65 Y (18-39, 40-64).
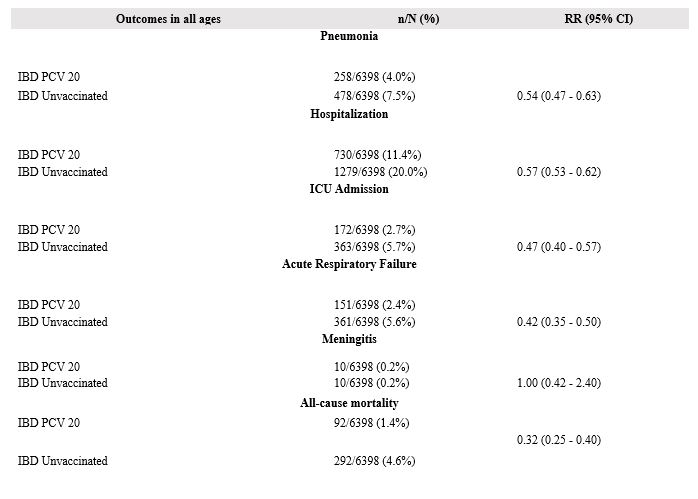
Results: After PSM, 12,796 patients were included in the analysis (Table 1). Compared to the control group, the vaccinated group had reduced risks of pneumonia (RR 0.54 [95% CI 0.47-0.63]), ARF (RR 0.42 [95% CI 0.35-0.50]), hospital admissions (RR 0.57 [95% CI 0.53-0.62]), ICU service utilization (RR 0.47 [95% CI 0.40-0.57]), all-cause mortality (RR 0.32 [95% CI 0.25-0.40]), but not meningitis (RR 1 [95% CI 0.42-2.40]) (Figure 1). Pneumonia risk remained lower in the vaccinated group aged 18-39 y (RR 0.50 [95% CI 0.29-0.84]) and 40-64 y (RR 0.55 [95% CI 0.43-0.70]).
Discussion: Our analysis revealed lower pneumonia risk and improved outcomes in patients with IBD associated with pneumococcal vaccines. It remains possible that vaccination could reflect better-received care, patient adherence, and motivation, which could influence the studied outcomes. Nonetheless, our analysis attempted to account for this through a rigorous propensity-matching process, resulting in largely balanced groups. Thus, our findings support the current pneumococcal vaccination recommendations for adult patients with IBD.
Disclosure: A supervised AI helped design the tables but was not included in the analysis process.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Mouhand F.H. Mohamed, MD, MSc1, Azizullah Beran, MD2, Osama Hamid, MD3, Francis A. Farraye, MD, MSc4, Samir A. Shah, MD5. P2616 - Pneumococcal Vaccine is Associated with Improved Outcomes in Inflammatory Bowel Disease: Insights From a Propensity-Matched Study in the United States, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2Indiana University School of Medicine, Indianapolis, IN; 3University of Texas Southwestern Medical Center, Dallas, TX; 4Mayo Clinic, Jacksonville, FL; 5Gastroenterology Associates, Inc., powered by GI Alliance, Providence, RI
Introduction: The pneumococcal vaccine is recommended for patients with inflammatory bowel disease (IBD) due to an increased risk of pneumonia. The aim of this study was to evaluate pneumonia related outcomes in patients with IBD who received pneumococcal vaccination.
Methods: We analyzed TriNetX US Collaborative Network data, including > 100 million patient records from 64 healthcare organizations. Patients with IBD age >18 were included (January 2021 to June 2024). Using ICD-10 codes, we included Ulcerative colitis (UC) or Crohn's disease (CD) with at least one IBD therapy. Due to its single-dose regimen, we only included the pneumococcal vaccine 20-valent (PCV20) in the study as a surrogate for complete pneumococcal vaccination. For the control group, we excluded all forms of pneumococcal vaccines. A propensity score matching (PSM) accounting for demographics and pneumonia risk factors. We assessed outcomes > 30 days after vaccine administration. The primary outcome was the pneumonia risk. Other outcomes included hospitalizations, intensive care unit (ICU) services, acute respiratory failure (ARF), and all-cause mortality. We generated relative risk (RR) with 95% confidence intervals (CI). We performed a sub-group analysis for the primary outcome in patients < 65 Y (18-39, 40-64).
Results: After PSM, 12,796 patients were included in the analysis (Table 1). Compared to the control group, the vaccinated group had reduced risks of pneumonia (RR 0.54 [95% CI 0.47-0.63]), ARF (RR 0.42 [95% CI 0.35-0.50]), hospital admissions (RR 0.57 [95% CI 0.53-0.62]), ICU service utilization (RR 0.47 [95% CI 0.40-0.57]), all-cause mortality (RR 0.32 [95% CI 0.25-0.40]), but not meningitis (RR 1 [95% CI 0.42-2.40]) (Figure 1). Pneumonia risk remained lower in the vaccinated group aged 18-39 y (RR 0.50 [95% CI 0.29-0.84]) and 40-64 y (RR 0.55 [95% CI 0.43-0.70]).
Discussion: Our analysis revealed lower pneumonia risk and improved outcomes in patients with IBD associated with pneumococcal vaccines. It remains possible that vaccination could reflect better-received care, patient adherence, and motivation, which could influence the studied outcomes. Nonetheless, our analysis attempted to account for this through a rigorous propensity-matching process, resulting in largely balanced groups. Thus, our findings support the current pneumococcal vaccination recommendations for adult patients with IBD.
Disclosure: A supervised AI helped design the tables but was not included in the analysis process.

Figure: Figure 1: Summary of the study outcomes
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Mouhand Mohamed indicated no relevant financial relationships.
Azizullah Beran indicated no relevant financial relationships.
Osama Hamid indicated no relevant financial relationships.
Francis Farraye: AbbVie – Consultant. Avalo Therapeutics – Consultant. Bausch – Advisor or Review Panel Member. BMS – Consultant. Braintree Labs – Consultant. DSMB for Lilly. – Sits on. Fresenius Kabi – Consultant. GI Reviewers and IBD Educational Group – independent contractor. GSK, Iterative Health, Janssen, Pfizer, Pharmacosmos, Sandoz Immunology, Sebela and Viatris – Consultant.
Samir Shah: Roche Information Systems – Consultant.
Mouhand F.H. Mohamed, MD, MSc1, Azizullah Beran, MD2, Osama Hamid, MD3, Francis A. Farraye, MD, MSc4, Samir A. Shah, MD5. P2616 - Pneumococcal Vaccine is Associated with Improved Outcomes in Inflammatory Bowel Disease: Insights From a Propensity-Matched Study in the United States, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
