Monday Poster Session
Category: IBD
P2621 - Evaluating the Safety of JAK Inhibitors in Ulcerative Colitis Patients Undergoing Colectomy: A Propensity Score-Matched Analysis
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E

Abdullah Sohail, MD
The University of Iowa Hospitals and Clinics
Iowa City, IA
Presenting Author(s)
Abdullah Sohail, MD1, Preetika Sinh, MD2, Muhammad Bhinder, MD3, Gregory Cooper, MD4, Vu Q. Nguyen, MD, MS4, Jeffry Katz, MD4, Fabio Cominelli, MD, PhD4, Miguel D Regueiro, MD5, Emad Mansoor, MD4
1The University of Iowa Hospitals and Clinics, Iowa City, IA; 2Medical College of Wisconsin, Milwaukee, WI; 3Charleston Area Medical Center, Charleston, WV; 4Digestive Health Institute, University Hospitals Cleveland Medical Center, Cleveland, OH; 5Cleveland Clinic, Cleveland, OH
Introduction: Patients with ulcerative colitis (UC) undergoing total colectomy are at a high risk of significant postoperative morbidity, including venous thromboembolism (VTE), with a 2-to 4-fold increased risk of thromboembolic events. Recent concerns about major adverse cardiovascular events (MACE), VTE, and infections associated with Janus Kinase (JAKi) inhibitors necessitate the evaluation of these postoperative risks. This study examined the impact of presurgical JAKi use on these postoperative risks in patients with UC undergoing colectomy.
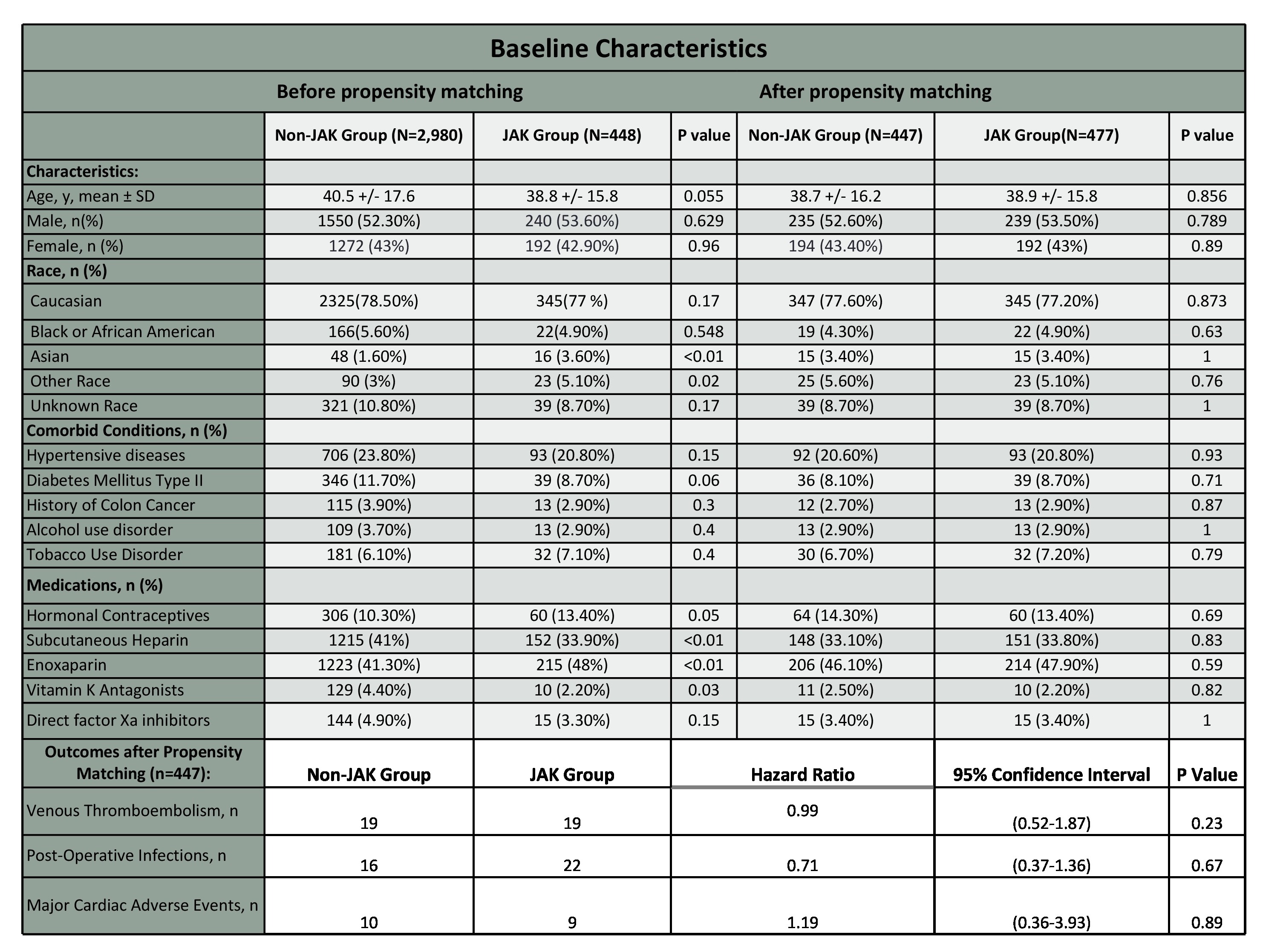
Methods: This retrospective cohort study and time-to-event analysis used data from the TriNetX network (May 30, 2018, to May 30, 2024). This study included patients aged >18 years with moderate-to-severe UC who underwent total colectomy. The patients were categorized into two groups: the non-JAK group (treated with anti-TNF agents, integrin receptor antagonists, and anti-IL-12/23 agents) and the JAK inhibitor group (treated with tofacitinib and upadacitinib). The exclusion criteria included hereditary thrombophilia or history of VTE. Propensity score matching was used to balance the groups by age, gender, race, comorbidities, and perioperative drug exposure. Primary outcomes assessed were 90-day postoperative VTE, MACE, and infections, compared using Cox proportional hazards regression models.
Results: We identified 2,980 patients in the non-JAKi group and 448 patients in the JAKi group. Following propensity score matching, 447 patients were included in each group. After matching, the analysis showed no significant differences in the primary outcomes between the two groups over the 90-day postoperative period. Specifically, the incidence of VTE was 4.25% in the non-JAK group compared to 4.50% in the JAK inhibitor group (P=0.23). Similarly, postoperative infections occurred in 3.57% of the non-JAK group and 4.92% of the JAK inhibitor group (P=0.67). The rate of MACE was 2.23% in the non-JAK group versus 2.30% in the JAK inhibitor group (P=0.89).
Discussion: Our study demonstrates that the risk of postoperative VTE, infections, and MACE in UC patients treated with JAK inhibitors undergoing colectomy is comparable to those treated with anti-TNF agents, integrin receptor antagonists, and anti-IL-12/23 agents. These findings indicate that JAK inhibitors do not confer additional postoperative risk in this high-risk population, supporting their safe use in clinical practice for patients with moderate to severe UC undergoing colectomy.
Disclosures:
Abdullah Sohail, MD1, Preetika Sinh, MD2, Muhammad Bhinder, MD3, Gregory Cooper, MD4, Vu Q. Nguyen, MD, MS4, Jeffry Katz, MD4, Fabio Cominelli, MD, PhD4, Miguel D Regueiro, MD5, Emad Mansoor, MD4. P2621 - Evaluating the Safety of JAK Inhibitors in Ulcerative Colitis Patients Undergoing Colectomy: A Propensity Score-Matched Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1The University of Iowa Hospitals and Clinics, Iowa City, IA; 2Medical College of Wisconsin, Milwaukee, WI; 3Charleston Area Medical Center, Charleston, WV; 4Digestive Health Institute, University Hospitals Cleveland Medical Center, Cleveland, OH; 5Cleveland Clinic, Cleveland, OH
Introduction: Patients with ulcerative colitis (UC) undergoing total colectomy are at a high risk of significant postoperative morbidity, including venous thromboembolism (VTE), with a 2-to 4-fold increased risk of thromboembolic events. Recent concerns about major adverse cardiovascular events (MACE), VTE, and infections associated with Janus Kinase (JAKi) inhibitors necessitate the evaluation of these postoperative risks. This study examined the impact of presurgical JAKi use on these postoperative risks in patients with UC undergoing colectomy.
Methods: This retrospective cohort study and time-to-event analysis used data from the TriNetX network (May 30, 2018, to May 30, 2024). This study included patients aged >18 years with moderate-to-severe UC who underwent total colectomy. The patients were categorized into two groups: the non-JAK group (treated with anti-TNF agents, integrin receptor antagonists, and anti-IL-12/23 agents) and the JAK inhibitor group (treated with tofacitinib and upadacitinib). The exclusion criteria included hereditary thrombophilia or history of VTE. Propensity score matching was used to balance the groups by age, gender, race, comorbidities, and perioperative drug exposure. Primary outcomes assessed were 90-day postoperative VTE, MACE, and infections, compared using Cox proportional hazards regression models.
Results: We identified 2,980 patients in the non-JAKi group and 448 patients in the JAKi group. Following propensity score matching, 447 patients were included in each group. After matching, the analysis showed no significant differences in the primary outcomes between the two groups over the 90-day postoperative period. Specifically, the incidence of VTE was 4.25% in the non-JAK group compared to 4.50% in the JAK inhibitor group (P=0.23). Similarly, postoperative infections occurred in 3.57% of the non-JAK group and 4.92% of the JAK inhibitor group (P=0.67). The rate of MACE was 2.23% in the non-JAK group versus 2.30% in the JAK inhibitor group (P=0.89).
Discussion: Our study demonstrates that the risk of postoperative VTE, infections, and MACE in UC patients treated with JAK inhibitors undergoing colectomy is comparable to those treated with anti-TNF agents, integrin receptor antagonists, and anti-IL-12/23 agents. These findings indicate that JAK inhibitors do not confer additional postoperative risk in this high-risk population, supporting their safe use in clinical practice for patients with moderate to severe UC undergoing colectomy.

Figure: Table 1: Baseline Characteristics and 90-day Outcomes of UC patients undergoing Colectomy
Disclosures:
Abdullah Sohail indicated no relevant financial relationships.
Preetika Sinh: Bristol Myers Squibb – Advisory Committee/Board Member.
Muhammad Bhinder indicated no relevant financial relationships.
Gregory Cooper indicated no relevant financial relationships.
Vu Nguyen: AbbVie – Speakers Bureau. Eli Lilly – Speakers Bureau.
Jeffry Katz indicated no relevant financial relationships.
Fabio Cominelli indicated no relevant financial relationships.
Miguel D Regueiro: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Emad Mansoor: Lilly – Speakers Bureau. Takeda – Speakers Bureau.
Abdullah Sohail, MD1, Preetika Sinh, MD2, Muhammad Bhinder, MD3, Gregory Cooper, MD4, Vu Q. Nguyen, MD, MS4, Jeffry Katz, MD4, Fabio Cominelli, MD, PhD4, Miguel D Regueiro, MD5, Emad Mansoor, MD4. P2621 - Evaluating the Safety of JAK Inhibitors in Ulcerative Colitis Patients Undergoing Colectomy: A Propensity Score-Matched Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
