Monday Poster Session
Category: GI Bleeding
P2468 - Role of Metoclopramide During Endoscopy in Patients With Upper Gastrointestinal Bleeding
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
.jpg)
Sahib Singh, MD
Sinai Hospital
Baltimore, MD
Presenting Author(s)
Sahib Singh, MD1, Ayushi Shah, MD2, Bhanu Siva Mohan Pinnam, MD3, Babu Mohan, MD4, Vishnu Charan Suresh Kumar, MD5, Ganesh Aswath, MD5, Rakesh Vinayek, MD1, Sudhir Dutta, MD1, Dushyant S. Dahiya, MD6, Sumant Inamdar, MD7, Douglas Adler, MD8, Hassam Ali, MD9, Neil R Sharma, MD, FACG10
1Sinai Hospital, Baltimore, MD; 2SUNY Upstate Medical University, Omaha, NE; 3John H. Stroger, Jr. Hospital of Cook County, Chicago, IL; 4Orlando Gastroenterology PA, Orlando, FL; 5SUNY Upstate Medical University, Syracuse, NY; 6The University of Kansas School of Medicine, Kansas City, KS; 7University of Arkansas for Medical Sciences, Little Rock, AR; 8Center for Advanced Therapeutic (CATE), Centura Health, Porter Adventist Hospital, Peak Gastroenterology, Denver, CO; 9ECU Health Medical Center, Greenville, NC; 10IOSE. Peak Gastroenterology & Gastrocare Partners., Colorado Springs, CO
Introduction: Metoclopramide, a prokinetic agent, has been shown to improve endoscopic mucosal visualization in patients with upper gastrointestinal bleeding (UGIB). However, the effect on clinical outcomes is unknown, with the studies reporting variable results. We performed a meta-analysis of the available data.
Methods: Online databases were searched for studies comparing metoclopramide to control (placebo or no medication) in UGIB patients undergoing esophagogastroduodenoscopy (EGD). The outcomes of interest were repeat EGD rate, patients requiring blood transfusions, mean blood units transfused, length of hospital stay (days), adverse events and mortality. Pooled odds ratios (OR) and standardized mean differences (SMD), along with 95% confidence intervals (CI) were calculated using a random effects model.
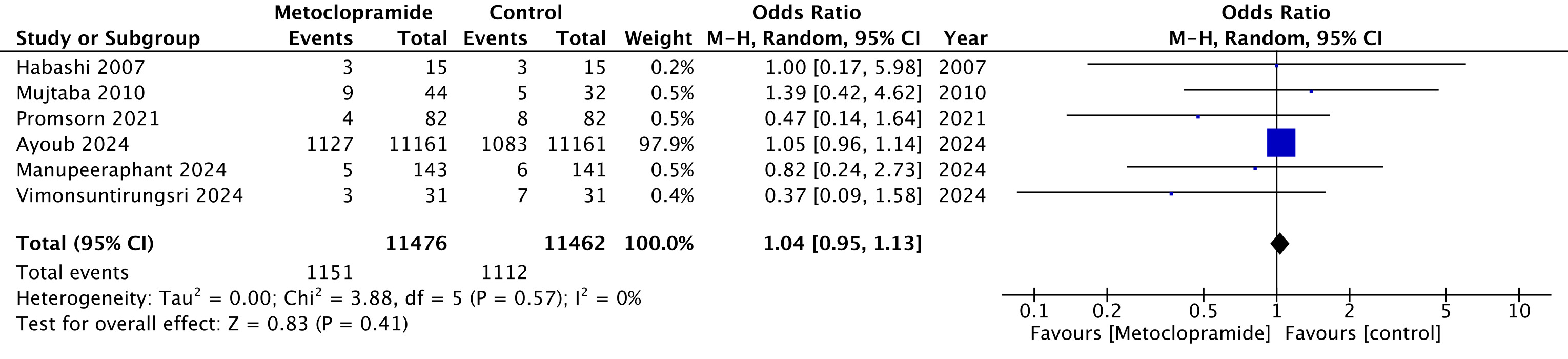
Results: A total of 6 studies (5 randomized controlled trials [RCTs] and 1 observational) with 22,944 patients (metoclopramide n=11,479, control n=11,465) were included. The mean age was 60 years and 59% of patients were men. No significant differences were observed between the metoclopramide and control groups with respect to repeat EGD rate (OR 1.04, 95% CI 0.95 to 1.13, p = 0.41) (Figure 1), patients needing blood transfusions (OR 0.90, 95% CI 0.68 to 1.18, p = 0.43), blood units transfused (SMD 0.03, 95% CI -0.14 to 0.21, p = 0.72), length of hospital stay (SMD -0.06, 95% CI -0.34 to 0.21, p = 0.64) and overall mortality (OR 0.97, 95% CI 0.76 to 1.24, p = 0.81). Metoclopramide use was not associated with any adverse events. The outcomes were consistent in subgroup analysis of only RCTs.
Discussion: As compared with the control group, using metoclopramide during endoscopy in patients with UGIB did not improve repeat EGD rate, need for blood transfusions or duration of hospitalization.
Disclosures:
Sahib Singh, MD1, Ayushi Shah, MD2, Bhanu Siva Mohan Pinnam, MD3, Babu Mohan, MD4, Vishnu Charan Suresh Kumar, MD5, Ganesh Aswath, MD5, Rakesh Vinayek, MD1, Sudhir Dutta, MD1, Dushyant S. Dahiya, MD6, Sumant Inamdar, MD7, Douglas Adler, MD8, Hassam Ali, MD9, Neil R Sharma, MD, FACG10. P2468 - Role of Metoclopramide During Endoscopy in Patients With Upper Gastrointestinal Bleeding, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Sinai Hospital, Baltimore, MD; 2SUNY Upstate Medical University, Omaha, NE; 3John H. Stroger, Jr. Hospital of Cook County, Chicago, IL; 4Orlando Gastroenterology PA, Orlando, FL; 5SUNY Upstate Medical University, Syracuse, NY; 6The University of Kansas School of Medicine, Kansas City, KS; 7University of Arkansas for Medical Sciences, Little Rock, AR; 8Center for Advanced Therapeutic (CATE), Centura Health, Porter Adventist Hospital, Peak Gastroenterology, Denver, CO; 9ECU Health Medical Center, Greenville, NC; 10IOSE. Peak Gastroenterology & Gastrocare Partners., Colorado Springs, CO
Introduction: Metoclopramide, a prokinetic agent, has been shown to improve endoscopic mucosal visualization in patients with upper gastrointestinal bleeding (UGIB). However, the effect on clinical outcomes is unknown, with the studies reporting variable results. We performed a meta-analysis of the available data.
Methods: Online databases were searched for studies comparing metoclopramide to control (placebo or no medication) in UGIB patients undergoing esophagogastroduodenoscopy (EGD). The outcomes of interest were repeat EGD rate, patients requiring blood transfusions, mean blood units transfused, length of hospital stay (days), adverse events and mortality. Pooled odds ratios (OR) and standardized mean differences (SMD), along with 95% confidence intervals (CI) were calculated using a random effects model.
Results: A total of 6 studies (5 randomized controlled trials [RCTs] and 1 observational) with 22,944 patients (metoclopramide n=11,479, control n=11,465) were included. The mean age was 60 years and 59% of patients were men. No significant differences were observed between the metoclopramide and control groups with respect to repeat EGD rate (OR 1.04, 95% CI 0.95 to 1.13, p = 0.41) (Figure 1), patients needing blood transfusions (OR 0.90, 95% CI 0.68 to 1.18, p = 0.43), blood units transfused (SMD 0.03, 95% CI -0.14 to 0.21, p = 0.72), length of hospital stay (SMD -0.06, 95% CI -0.34 to 0.21, p = 0.64) and overall mortality (OR 0.97, 95% CI 0.76 to 1.24, p = 0.81). Metoclopramide use was not associated with any adverse events. The outcomes were consistent in subgroup analysis of only RCTs.
Discussion: As compared with the control group, using metoclopramide during endoscopy in patients with UGIB did not improve repeat EGD rate, need for blood transfusions or duration of hospitalization.

Figure: Repeat upper endoscopy rate
Disclosures:
Sahib Singh indicated no relevant financial relationships.
Ayushi Shah indicated no relevant financial relationships.
Bhanu Siva Mohan Pinnam indicated no relevant financial relationships.
Babu Mohan indicated no relevant financial relationships.
Vishnu Charan Suresh Kumar indicated no relevant financial relationships.
Ganesh Aswath indicated no relevant financial relationships.
Rakesh Vinayek indicated no relevant financial relationships.
Sudhir Dutta indicated no relevant financial relationships.
Dushyant Dahiya indicated no relevant financial relationships.
Sumant Inamdar indicated no relevant financial relationships.
Douglas Adler: Boston Scientific and Micro Tech. – Consultant.
Hassam Ali indicated no relevant financial relationships.
Neil R Sharma: Boston Scientific – Consultant. Medtronic – Consultant. Olympus – Consultant. Steris – Consultant.
Sahib Singh, MD1, Ayushi Shah, MD2, Bhanu Siva Mohan Pinnam, MD3, Babu Mohan, MD4, Vishnu Charan Suresh Kumar, MD5, Ganesh Aswath, MD5, Rakesh Vinayek, MD1, Sudhir Dutta, MD1, Dushyant S. Dahiya, MD6, Sumant Inamdar, MD7, Douglas Adler, MD8, Hassam Ali, MD9, Neil R Sharma, MD, FACG10. P2468 - Role of Metoclopramide During Endoscopy in Patients With Upper Gastrointestinal Bleeding, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
