Monday Poster Session
Category: Colon
P1915 - Safety of Fecal Microbiota Spores, Live-brpk (Formerly SER-109) in Participants With Recurrent Clostridioides difficile Infection and Comorbidities: Findings From an Integrated Analysis of Phase 3 Trials
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- MS
Matthew Sims, MD, PhD
Corewell Health William Beaumont University Hospital
Royal Oak, MI
Presenting Author(s)
Matthew Sims, MD, PhD1, Charles S.. Berenson, MD2, Robert Stevens, PharmD3, Dianne Nguyen, MD, MBA4, John R. Pullman, MD5
1Corewell Health William Beaumont University Hospital, Royal Oak, MI; 2University at Buffalo, VA Western New York Healthcare System, Buffalo, NY; 3Seres Therapeutics, Cambridge, MA; 4Nestlé Health Science, Bridgewater, NJ; 5Mercury Street Medical Group, PLLC, Butte, MT
Introduction: Clinically relevant comorbid conditions increase risk of recurrent Clostridioides difficile infection (rCDI) with potentially more severe clinical course or consequences. Fecal microbiota spores, live-brpk (VOWST™; formerly SER-109, hereafter referred to as VOS) is an orally administered microbiome therapeutic to prevent future recurrence of CDI in adults after antibiotic treatment for rCDI. This integrated analysis of two Phase 3 trials further describes VOS safety in participants (pts) with select comorbidities.
Methods: The randomized, double-blind, placebo-controlled ECOSPOR III trial enrolled 182 pts with ≥2 CDI recurrences; the open-label, single-arm ECOSPOR IV trial enrolled 263 pts with rCDI. VOS was given orally as 4 capsules daily for 3 consecutive days after completion of antibiotics. Treatment-emergent adverse events (TEAEs) were obtained through Week 8; serious TEAEs/AEs of special interest were obtained through Week 24. Treatment-related adverse events (TRAEs) were TEAEs considered related/possibly related to study drug. VOS data from the integrated analysis and placebo data from ECOSPOR III are reported by comorbidity subgroups.
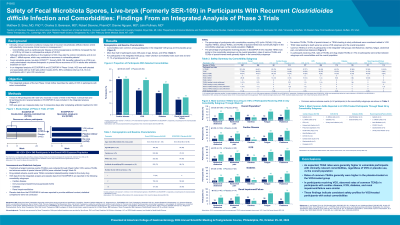
Results: Pts with comorbidities receiving VOS (n=178/349, 51%) reported numerically higher rates of TEAEs, serious TEAEs, and TEAEs of special interest in renal impairment/failure (80%, 35%, and 26%, respectively), diabetes (70%, 20%, and 9%), cardiac disease (75%, 27%, and 12%), and immunocompromised/immunosuppressed (IC/IS; 77%, 20%, and 14%) subgroups vs the overall population (Table). Pts receiving placebo in ECOSPOR III reported similar rates of TEAEs, serious TEAEs, and TEAEs of special interest in renal impairment/failure (100%, 57%, and 0%, respectively), diabetes (80%, 32%, and 8%), cardiac disease (97%, 41%, and 10%), and IC/IS (93%, 32%, and 4%) subgroups. In pts receiving VOS, TRAE rates were similar between subgroups with comorbidities and the overall population. No serious TEAEs, TEAEs of special interest, or TEAEs leading to study withdrawal were considered related to VOS. TEAE rates leading to death were low across VOS subgroups and the overall integrated population.
Discussion: As expected, TEAE rates were generally higher in vulnerable pts with clinically relevant comorbidities, regardless of VOS or placebo use, vs the overall populations. In pts receiving VOS, TRAE rates were generally low across subgroups and the overall population, indicating similar safety profiles for VOS in all pts.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Matthew Sims, MD, PhD1, Charles S.. Berenson, MD2, Robert Stevens, PharmD3, Dianne Nguyen, MD, MBA4, John R. Pullman, MD5. P1915 - Safety of Fecal Microbiota Spores, Live-brpk (Formerly SER-109) in Participants With Recurrent <i>Clostridioides difficile</i> Infection and Comorbidities: Findings From an Integrated Analysis of Phase 3 Trials, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Corewell Health William Beaumont University Hospital, Royal Oak, MI; 2University at Buffalo, VA Western New York Healthcare System, Buffalo, NY; 3Seres Therapeutics, Cambridge, MA; 4Nestlé Health Science, Bridgewater, NJ; 5Mercury Street Medical Group, PLLC, Butte, MT
Introduction: Clinically relevant comorbid conditions increase risk of recurrent Clostridioides difficile infection (rCDI) with potentially more severe clinical course or consequences. Fecal microbiota spores, live-brpk (VOWST™; formerly SER-109, hereafter referred to as VOS) is an orally administered microbiome therapeutic to prevent future recurrence of CDI in adults after antibiotic treatment for rCDI. This integrated analysis of two Phase 3 trials further describes VOS safety in participants (pts) with select comorbidities.
Methods: The randomized, double-blind, placebo-controlled ECOSPOR III trial enrolled 182 pts with ≥2 CDI recurrences; the open-label, single-arm ECOSPOR IV trial enrolled 263 pts with rCDI. VOS was given orally as 4 capsules daily for 3 consecutive days after completion of antibiotics. Treatment-emergent adverse events (TEAEs) were obtained through Week 8; serious TEAEs/AEs of special interest were obtained through Week 24. Treatment-related adverse events (TRAEs) were TEAEs considered related/possibly related to study drug. VOS data from the integrated analysis and placebo data from ECOSPOR III are reported by comorbidity subgroups.
Results: Pts with comorbidities receiving VOS (n=178/349, 51%) reported numerically higher rates of TEAEs, serious TEAEs, and TEAEs of special interest in renal impairment/failure (80%, 35%, and 26%, respectively), diabetes (70%, 20%, and 9%), cardiac disease (75%, 27%, and 12%), and immunocompromised/immunosuppressed (IC/IS; 77%, 20%, and 14%) subgroups vs the overall population (Table). Pts receiving placebo in ECOSPOR III reported similar rates of TEAEs, serious TEAEs, and TEAEs of special interest in renal impairment/failure (100%, 57%, and 0%, respectively), diabetes (80%, 32%, and 8%), cardiac disease (97%, 41%, and 10%), and IC/IS (93%, 32%, and 4%) subgroups. In pts receiving VOS, TRAE rates were similar between subgroups with comorbidities and the overall population. No serious TEAEs, TEAEs of special interest, or TEAEs leading to study withdrawal were considered related to VOS. TEAE rates leading to death were low across VOS subgroups and the overall integrated population.
Discussion: As expected, TEAE rates were generally higher in vulnerable pts with clinically relevant comorbidities, regardless of VOS or placebo use, vs the overall populations. In pts receiving VOS, TRAE rates were generally low across subgroups and the overall population, indicating similar safety profiles for VOS in all pts.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Matthew Sims: Adaptive Phage Therapeutics – Grant/Research Support. Applied BioCode – Consultant, Grant/Research Support. AstraZeneca – Grant/Research Support. Biotest AG – Grant/Research Support. Crestone – Grant/Research Support. Dompe – Grant/Research Support. Janssen Research & Development, LLC – Grant/Research Support. Leonard-Meron Biosciences – Grant/Research Support. OpGen – Grant/Research Support. Pfizer – Grant/Research Support. PhAST – Grant/Research Support. Prenosis – Advisory Committee/Board Member. Prenosis – Grant/Research Support. QIAGEN – Grant/Research Support. Seres Therapeutics – Grant/Research Support. Venatorx Pharmaceuticals – Advisory Committee/Board Member.
Charles Berenson indicated no relevant financial relationships.
Robert Stevens: Seres Therapeutics – Employee, Stock-publicly held company(excluding mutual/index funds).
Dianne Nguyen: Abbvie – Stock-publicly held company(excluding mutual/index funds). bristol meyers squibb – Stock-publicly held company(excluding mutual/index funds). Nestle Health Science – Employee, Stock-publicly held company(excluding mutual/index funds).
John Pullman indicated no relevant financial relationships.
Matthew Sims, MD, PhD1, Charles S.. Berenson, MD2, Robert Stevens, PharmD3, Dianne Nguyen, MD, MBA4, John R. Pullman, MD5. P1915 - Safety of Fecal Microbiota Spores, Live-brpk (Formerly SER-109) in Participants With Recurrent <i>Clostridioides difficile</i> Infection and Comorbidities: Findings From an Integrated Analysis of Phase 3 Trials, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
