Monday Poster Session
Category: Colon
P1917 - Real-World Use of Fecal Microbiota Spores, Live-brpk (Formerly SER-109) in Adults With Recurrent Clostridioides Difficile Infection in the United States
Monday, October 28, 2024
10:30 AM - 4:00 PM ET
Location: Exhibit Hall E
Has Audio
- SA
Stuart Akerman, MD
Digestive Health Associates of Texas
Plano, TX
Presenting Author(s)
Stuart Akerman, MD1, Ashish Gumaste2, Michael Chen, MBA3, Lisa Connor, PharmD, MBA4, Maximilian von Eynatten, MD5, Jane De Vries, MSc6, Dianne Nguyen, MD, MBA2
1Digestive Health Associates of Texas, Plano, TX; 2Nestlé Health Science, Bridgewater, NJ; 3Nestle Health Science, Dallas, TX; 4Nestle Health Science, Lavallette, NJ; 5Nestle Health Science, Lausanne, Vaud, Switzerland; 6Independent Health Economist, Bagshot, England, United Kingdom
Introduction: Fecal microbiota spores, live-brpk (VOWST™; formerly SER-109, hereafter referred to as VOS) is an FDA-approved orally administered microbiome therapeutic to prevent recurrent Clostridioides difficile infection (rCDI) in adults after antibiotics for rCDI. VOS, taken as 4 capsules daily for 3 consecutive days, significantly reduced rCDI risk (ie, rCDI within 8 wks after study drug) vs placebo after standard-of-care antibiotics in adults with rCDI in the randomized, double-blind, placebo-controlled ECOSPOR III trial by 68% (rCDI rate: VOS, 12% vs placebo, 40%; relative risk, 0.32; 95% CI, 0.18–0.58; P< 0.001) (N Engl J Med. 2022;386:220-229). Pivotal trials evaluated VOS as single use to prevent rCDI; we study real-world use of VOS.
Methods: This retrospective, observational analysis assessed patient (pt) and dispensing data from adults with rCDI prescribed VOS and who received VOS from a limited network of specialty pharmacies in the United States. Demographics and clinical characteristics were also obtained from the pt support program, in which pts had the option to enroll for assistance acquiring VOS. Data were analyzed from June 5, 2023, to May 31, 2024. Proportions of pts with single or repeat VOS prescriptions were identified. Data were analyzed descriptively.
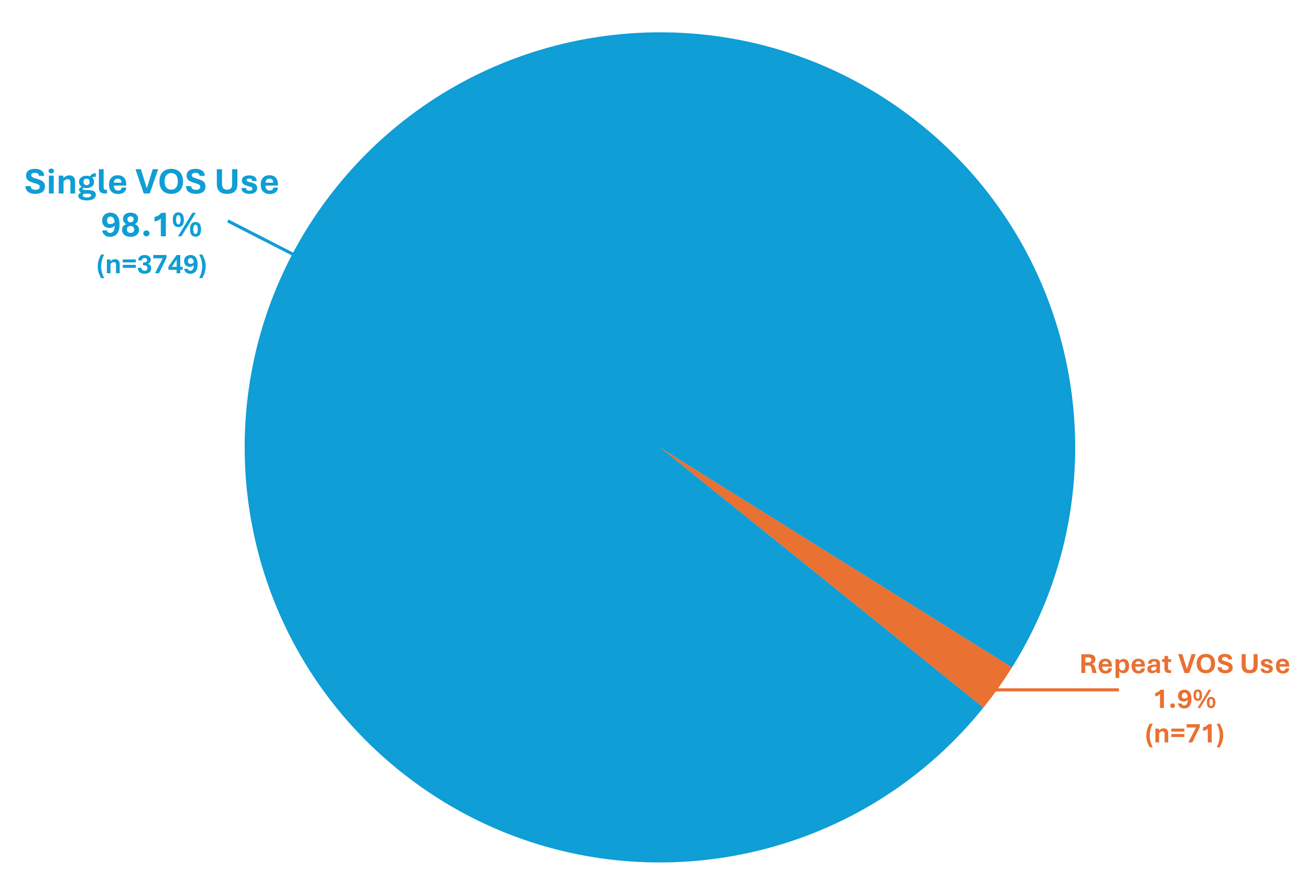
Results: Of 3820 unique pts receiving VOS, 1.9% (n=71) received repeat VOS prescriptions (Figure). Mean age for pts with single and repeat VOS use was 67.8 and 74.3 years, respectively. Most frequently (≥20%) prescribed antibiotics for rCDI prior to single vs repeat VOS use were vancomycin (49.1% vs 53.0%, respectively) and fidaxomicin (26.2% vs 25.5%). A subsequent VOS prescription was received ≤8 wks of the prior VOS use in 0.5% (n=21) of pts and after 8 wks in 1.3% (n=50) of pts. Median number of wks between consecutive VOS uses was 13.
Discussion: In the largest dataset of real-world VOS use available to date, over 98% of pts received VOS once to prevent recurrence of CDI after antibiotics for rCDI, in accordance with the drug’s intended single use. Limitations of our study include inability to determine reasons for repeat VOS prescriptions or to capture enrolled individuals not receiving VOS. Of pts receiving repeat VOS, most received it beyond 8 wks, suggesting new/distinct infection. These observations reveal VOS effectiveness for preventing rCDI in the real world and support/contextualize efficacy of VOS in clinical trials.
Disclosures:
Stuart Akerman, MD1, Ashish Gumaste2, Michael Chen, MBA3, Lisa Connor, PharmD, MBA4, Maximilian von Eynatten, MD5, Jane De Vries, MSc6, Dianne Nguyen, MD, MBA2. P1917 - Real-World Use of Fecal Microbiota Spores, Live-brpk (Formerly SER-109) in Adults With Recurrent <i>Clostridioides Difficile</i> Infection in the United States, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Digestive Health Associates of Texas, Plano, TX; 2Nestlé Health Science, Bridgewater, NJ; 3Nestle Health Science, Dallas, TX; 4Nestle Health Science, Lavallette, NJ; 5Nestle Health Science, Lausanne, Vaud, Switzerland; 6Independent Health Economist, Bagshot, England, United Kingdom
Introduction: Fecal microbiota spores, live-brpk (VOWST™; formerly SER-109, hereafter referred to as VOS) is an FDA-approved orally administered microbiome therapeutic to prevent recurrent Clostridioides difficile infection (rCDI) in adults after antibiotics for rCDI. VOS, taken as 4 capsules daily for 3 consecutive days, significantly reduced rCDI risk (ie, rCDI within 8 wks after study drug) vs placebo after standard-of-care antibiotics in adults with rCDI in the randomized, double-blind, placebo-controlled ECOSPOR III trial by 68% (rCDI rate: VOS, 12% vs placebo, 40%; relative risk, 0.32; 95% CI, 0.18–0.58; P< 0.001) (N Engl J Med. 2022;386:220-229). Pivotal trials evaluated VOS as single use to prevent rCDI; we study real-world use of VOS.
Methods: This retrospective, observational analysis assessed patient (pt) and dispensing data from adults with rCDI prescribed VOS and who received VOS from a limited network of specialty pharmacies in the United States. Demographics and clinical characteristics were also obtained from the pt support program, in which pts had the option to enroll for assistance acquiring VOS. Data were analyzed from June 5, 2023, to May 31, 2024. Proportions of pts with single or repeat VOS prescriptions were identified. Data were analyzed descriptively.
Results: Of 3820 unique pts receiving VOS, 1.9% (n=71) received repeat VOS prescriptions (Figure). Mean age for pts with single and repeat VOS use was 67.8 and 74.3 years, respectively. Most frequently (≥20%) prescribed antibiotics for rCDI prior to single vs repeat VOS use were vancomycin (49.1% vs 53.0%, respectively) and fidaxomicin (26.2% vs 25.5%). A subsequent VOS prescription was received ≤8 wks of the prior VOS use in 0.5% (n=21) of pts and after 8 wks in 1.3% (n=50) of pts. Median number of wks between consecutive VOS uses was 13.
Discussion: In the largest dataset of real-world VOS use available to date, over 98% of pts received VOS once to prevent recurrence of CDI after antibiotics for rCDI, in accordance with the drug’s intended single use. Limitations of our study include inability to determine reasons for repeat VOS prescriptions or to capture enrolled individuals not receiving VOS. Of pts receiving repeat VOS, most received it beyond 8 wks, suggesting new/distinct infection. These observations reveal VOS effectiveness for preventing rCDI in the real world and support/contextualize efficacy of VOS in clinical trials.

Figure: Single or repeat VOS use following antibiotic treatment for rCDI for prevention of future recurrence of CDI.
Abbreviations: CDI, Clostridioides difficile infection; rCDI, recurrent CDI; VOS, fecal microbiota spores, live-brpk (VOWST™; formerly SER-109).
Abbreviations: CDI, Clostridioides difficile infection; rCDI, recurrent CDI; VOS, fecal microbiota spores, live-brpk (VOWST™; formerly SER-109).
Disclosures:
Stuart Akerman: Nestle Health/Aimmune – Speakers Bureau.
Ashish Gumaste: Nestle Health Sciences – Employee.
Michael Chen: Nestlé Health Science – Employee.
Lisa Connor: Nestle Health Science – Employee.
Maximilian von Eynatten: Nestle Health Science – Employee.
Jane De Vries: Nestle Health Science – Independent Contractor.
Dianne Nguyen: Abbvie – Stock-publicly held company(excluding mutual/index funds). bristol meyers squibb – Stock-publicly held company(excluding mutual/index funds). Nestle Health Science – Employee, Stock-publicly held company(excluding mutual/index funds).
Stuart Akerman, MD1, Ashish Gumaste2, Michael Chen, MBA3, Lisa Connor, PharmD, MBA4, Maximilian von Eynatten, MD5, Jane De Vries, MSc6, Dianne Nguyen, MD, MBA2. P1917 - Real-World Use of Fecal Microbiota Spores, Live-brpk (Formerly SER-109) in Adults With Recurrent <i>Clostridioides Difficile</i> Infection in the United States, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
