Sunday Poster Session
Category: Colon
P0216 - FOLFOXIRI Plus Bevacizumab Versus FOLFOX/FOLFIRI Plus Bevacizumab for Metastatic Colorectal Cancer: An Updated Meta-Analysis
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio

Anum Khakwani, MD
Charleston Area Medical Center
Winterville, NC
Presenting Author(s)
Fnu Muhibullah, MD, MBBS1, Nouman Shafique, MD2, Anum Khakwani, MD3, Abdul Qadeer, 4, Shammas Farooq Bajwa, MD2, Amna Ehsan, MBBS5, Wajeeha Aiman, MD6
1West Virginia University Camden Clark Medical Center, Parkersburg, WV; 2AdventHealth, Orlando, FL; 3Charleston Area Medical Center, Winterville, NC; 4Nishtar Medical University, Multan, Punjab, Pakistan; 5Nishtar Medical University, Parkersburg, WV; 6New York Medical College - Saint Michael's Medical Center, Newark, NJ
Introduction: Colorectal cancer (CRC) is the third most common malignancy and the second most common cause of death in males. Over half of the patients with CRC develop metastasis, which worsens the prognosis. Resection of metastases has been shown to significantly improve survival among CRC patients. For inoperable disease, first-line treatment for metastatic CRC is usually a combination of cytotoxic drugs with a biologic, such as bevacizumab (Bev). Previous studies have favored triplet chemotherapy (FOLFOXIRI) plus Bev over doublet chemotherapy (FOLFOX or FOLFIRI) plus Bev for metastatic CRC, but a consensus has yet to be reached. In this updated meta-analysis, we aim to provide a comparison of the efficacy of FOLFOXIRI plus Bev with FOLFOX/FOLFIRI plus Bev.
Methods: We searched various databases from their inception until June 2024. We included studies comparing the efficacy of triplet chemotherapy plus Bev with doublet chemotherapy plus Bev in mCRC. Efficacy outcomes included progression-free survival (PFS), overall survival (OS) at a 12-month interval, and adverse effects (grade ≥3). Data were pooled using a random effects model and analyzed using RevMan 5.4 software. Odds ratios (OR) with a 95% confidence interval (CI) were reported.
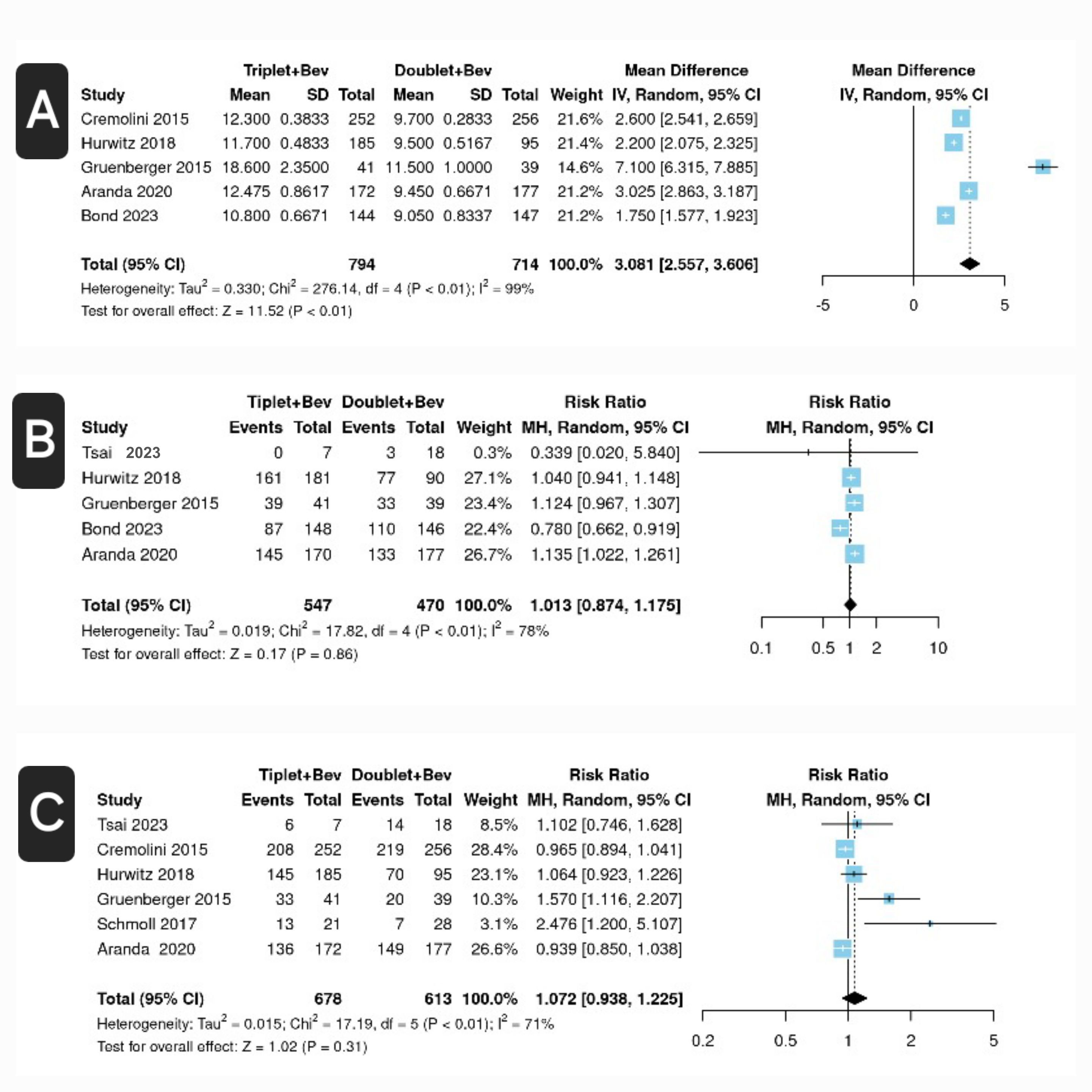
Results: Seven full-text studies met the inclusion criteria, with six of them being randomized controlled trials and one being an observational study. The total patient population was 1586. Compared to the FOLFOX/FOLFIRI plus Bev group, the FOLFOXIRI plus Bev group showed higher PFS [odds ratio (OR) 3.08, confidence interval (CI) 2.557-3.606, P< 0.01, I2 = 96%]. The FOLFOXIRI plus Bev group had a slightly higher risk of developing grade ≥3 adverse effects, but this association was not significant [OR 1.013 CI 0.874-1.174; p = 0.86; I2 = 78%]. The FOLFOXIRI plus Bev group also had a slightly higher overall survival rate, but this association was also not significant [OR 1.072, CI 0.938-1.225, p = 0.31, I2 = 71%].
Discussion: In this updated meta-analysis, we were able to demonstrate that FOLFOXIRI plus Bev can significantly increase PFS in patients with metastatic CRC. PFS is an important clinical endpoint in patients with advanced malignancies. However, results for OS at 12 months and adverse events grade ≥3 could not demonstrate the superiority of one type of treatment over the other.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Fnu Muhibullah, MD, MBBS1, Nouman Shafique, MD2, Anum Khakwani, MD3, Abdul Qadeer, 4, Shammas Farooq Bajwa, MD2, Amna Ehsan, MBBS5, Wajeeha Aiman, MD6. P0216 - FOLFOXIRI Plus Bevacizumab Versus FOLFOX/FOLFIRI Plus Bevacizumab for Metastatic Colorectal Cancer: An Updated Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1West Virginia University Camden Clark Medical Center, Parkersburg, WV; 2AdventHealth, Orlando, FL; 3Charleston Area Medical Center, Winterville, NC; 4Nishtar Medical University, Multan, Punjab, Pakistan; 5Nishtar Medical University, Parkersburg, WV; 6New York Medical College - Saint Michael's Medical Center, Newark, NJ
Introduction: Colorectal cancer (CRC) is the third most common malignancy and the second most common cause of death in males. Over half of the patients with CRC develop metastasis, which worsens the prognosis. Resection of metastases has been shown to significantly improve survival among CRC patients. For inoperable disease, first-line treatment for metastatic CRC is usually a combination of cytotoxic drugs with a biologic, such as bevacizumab (Bev). Previous studies have favored triplet chemotherapy (FOLFOXIRI) plus Bev over doublet chemotherapy (FOLFOX or FOLFIRI) plus Bev for metastatic CRC, but a consensus has yet to be reached. In this updated meta-analysis, we aim to provide a comparison of the efficacy of FOLFOXIRI plus Bev with FOLFOX/FOLFIRI plus Bev.
Methods: We searched various databases from their inception until June 2024. We included studies comparing the efficacy of triplet chemotherapy plus Bev with doublet chemotherapy plus Bev in mCRC. Efficacy outcomes included progression-free survival (PFS), overall survival (OS) at a 12-month interval, and adverse effects (grade ≥3). Data were pooled using a random effects model and analyzed using RevMan 5.4 software. Odds ratios (OR) with a 95% confidence interval (CI) were reported.
Results: Seven full-text studies met the inclusion criteria, with six of them being randomized controlled trials and one being an observational study. The total patient population was 1586. Compared to the FOLFOX/FOLFIRI plus Bev group, the FOLFOXIRI plus Bev group showed higher PFS [odds ratio (OR) 3.08, confidence interval (CI) 2.557-3.606, P< 0.01, I2 = 96%]. The FOLFOXIRI plus Bev group had a slightly higher risk of developing grade ≥3 adverse effects, but this association was not significant [OR 1.013 CI 0.874-1.174; p = 0.86; I2 = 78%]. The FOLFOXIRI plus Bev group also had a slightly higher overall survival rate, but this association was also not significant [OR 1.072, CI 0.938-1.225, p = 0.31, I2 = 71%].
Discussion: In this updated meta-analysis, we were able to demonstrate that FOLFOXIRI plus Bev can significantly increase PFS in patients with metastatic CRC. PFS is an important clinical endpoint in patients with advanced malignancies. However, results for OS at 12 months and adverse events grade ≥3 could not demonstrate the superiority of one type of treatment over the other.

Figure: Figure: Forest plots for FOLFOXIRI + Bevacizumab (Triplet + Bev) versus FOLFOX/FOLFIRI + Bevacizumab (Doublet + Bev) in metastatic colorectal carcinoma (a) mean progression-free survival (months) (b) adverse events (grade ≥ 3) (c) overall survival at a 12-month interval
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Fnu Muhibullah indicated no relevant financial relationships.
Nouman Shafique indicated no relevant financial relationships.
Anum Khakwani indicated no relevant financial relationships.
Abdul Qadeer indicated no relevant financial relationships.
Shammas Farooq Bajwa indicated no relevant financial relationships.
Amna Ehsan indicated no relevant financial relationships.
Wajeeha Aiman indicated no relevant financial relationships.
Fnu Muhibullah, MD, MBBS1, Nouman Shafique, MD2, Anum Khakwani, MD3, Abdul Qadeer, 4, Shammas Farooq Bajwa, MD2, Amna Ehsan, MBBS5, Wajeeha Aiman, MD6. P0216 - FOLFOXIRI Plus Bevacizumab Versus FOLFOX/FOLFIRI Plus Bevacizumab for Metastatic Colorectal Cancer: An Updated Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
