Sunday Poster Session
Category: Colorectal Cancer Prevention
P0427 - A Post Hoc Safety Analysis of Flavored Polyethylene Glycol and Sulfate Solution Bowel Preparation in Elderly Patients and Patients With Renal Insufficiency
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
.jpg)
Michael E. Beckelic, BSN
Braintree Laboratories, Inc.
Roswell, GA
Presenting Author(s)
Jack DiPalma, MD1, Raj Bhandari, MD2, Douglas Rex, MD3, Andrew Swanson, 4, Michael E.. Beckelic, BSN5
1University of South Alabama, Mobile, AL; 2Delta Research Partners, Monroe, LA; 3Indiana University School of Medicine, Indianapolis, IN; 4Braintree Laboratories, Inc., Braintree, MA; 5Braintree Laboratories, Inc., Roswell, GA
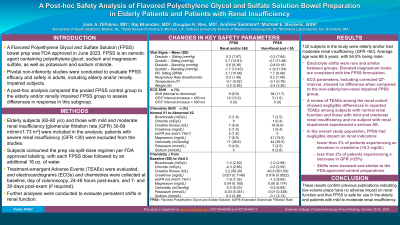
Introduction: A Flavored Polyethylene Glycol and Sulfate Solution (FPSS) bowel prep was FDA approved in June 2023. FPSS is an osmotic agent containing polyethylene glycol, sodium and magnesium sulfate, as well as potassium and sodium chloride. Pivotal studies in adults, including elderly and/or renally impaired subjects, were conducted to evaluate FPSS efficacy and safety in which FPSS established non-inferiority against comparative preps. A post-hoc analysis compared the pooled FPSS control group to the elderly and/or renally impaired FPSS group.
Methods: Elderly subjects (65-80 y/o) and those with mild and moderate renal insufficiency [glomerular filtration rate (GFR) 30-89 ml/min/1.73 m2] were included in the analysis; patients with severe renal insufficiency (GFR < 30) were excluded from the studies. Subjects consumed the prep via split-dose regimen per FDA approved labeling, with each FPSS dose being followed with an additional 16 oz. water. Electrocardiograms (ECGs) and chemistries were collected at baseline, day of colonoscopy, 24-48 hours post-exam, and 7 and 30 days post-exam (if required). Further analyses were conducted to evaluate persistent shifts in renal function. Treatment-Emergent Adverse Events (TEAEs) were collected within a cohort that included subjects with normal, mildly impaired and moderately impaired renal function.
Results: 132 subjects in the study were elderly and/or had moderate renal insufficiency (GFR < 60). Average age was 68.5 years, with 54.5% being male. Electrolyte shifts were rare and similar between groups. Elevated magnesium levels are consistent with the FPSS formulation. ECG parameters, including corrected QT-interval, showed no difference when compared to the non-elderly/non-renal impaired FPSS group. A review of TEAEs for the renal cohort showed negligible differences in reported TEAEs among subjects with normal renal function and those with mild and moderate renal insufficiency. No subject with renal impairment experienced a serious AE. In the overall study population, chemistry data confirmed that FPSS had negligible impact on renal indicators, with fewer than 3% of patients experiencing elevation in creatinine levels ( >0.3 mg/dL) and less than 2% of patients experiencing decrease in GFR ( >25%) following preparation. These results confirm previous publications indicating low-volume preps have no adverse impact on renal function.
Discussion: This post-hoc analysis confirms that FPSS is safe for use in the elderly and patients with mild to moderate renal insufficiency.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Jack DiPalma, MD1, Raj Bhandari, MD2, Douglas Rex, MD3, Andrew Swanson, 4, Michael E.. Beckelic, BSN5. P0427 - A <i>Post Hoc</i> Safety Analysis of Flavored Polyethylene Glycol and Sulfate Solution Bowel Preparation in Elderly Patients and Patients With Renal Insufficiency, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of South Alabama, Mobile, AL; 2Delta Research Partners, Monroe, LA; 3Indiana University School of Medicine, Indianapolis, IN; 4Braintree Laboratories, Inc., Braintree, MA; 5Braintree Laboratories, Inc., Roswell, GA
Introduction: A Flavored Polyethylene Glycol and Sulfate Solution (FPSS) bowel prep was FDA approved in June 2023. FPSS is an osmotic agent containing polyethylene glycol, sodium and magnesium sulfate, as well as potassium and sodium chloride. Pivotal studies in adults, including elderly and/or renally impaired subjects, were conducted to evaluate FPSS efficacy and safety in which FPSS established non-inferiority against comparative preps. A post-hoc analysis compared the pooled FPSS control group to the elderly and/or renally impaired FPSS group.
Methods: Elderly subjects (65-80 y/o) and those with mild and moderate renal insufficiency [glomerular filtration rate (GFR) 30-89 ml/min/1.73 m2] were included in the analysis; patients with severe renal insufficiency (GFR < 30) were excluded from the studies. Subjects consumed the prep via split-dose regimen per FDA approved labeling, with each FPSS dose being followed with an additional 16 oz. water. Electrocardiograms (ECGs) and chemistries were collected at baseline, day of colonoscopy, 24-48 hours post-exam, and 7 and 30 days post-exam (if required). Further analyses were conducted to evaluate persistent shifts in renal function. Treatment-Emergent Adverse Events (TEAEs) were collected within a cohort that included subjects with normal, mildly impaired and moderately impaired renal function.
Results: 132 subjects in the study were elderly and/or had moderate renal insufficiency (GFR < 60). Average age was 68.5 years, with 54.5% being male. Electrolyte shifts were rare and similar between groups. Elevated magnesium levels are consistent with the FPSS formulation. ECG parameters, including corrected QT-interval, showed no difference when compared to the non-elderly/non-renal impaired FPSS group. A review of TEAEs for the renal cohort showed negligible differences in reported TEAEs among subjects with normal renal function and those with mild and moderate renal insufficiency. No subject with renal impairment experienced a serious AE. In the overall study population, chemistry data confirmed that FPSS had negligible impact on renal indicators, with fewer than 3% of patients experiencing elevation in creatinine levels ( >0.3 mg/dL) and less than 2% of patients experiencing decrease in GFR ( >25%) following preparation. These results confirm previous publications indicating low-volume preps have no adverse impact on renal function.
Discussion: This post-hoc analysis confirms that FPSS is safe for use in the elderly and patients with mild to moderate renal insufficiency.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Jack DiPalma: Sebela Pharmaceuticals – Consultant, Independent Contractor.
Raj Bhandari indicated no relevant financial relationships.
Douglas Rex: Acacia Pharmaceuticals – Consultant. Boston Scientific – Consultant. Braintree Laboratories – Consultant, Grant/Research Support. Erbe USA – Grant/Research Support. Medivators – Grant/Research Support. Medtronic – Consultant. Norgine – Consultant. Olympus Corporation – Consultant, Grant/Research Support. Satisfai Health – Stock Options.
Andrew Swanson: Braintree Laboratories, Inc. – Employee.
Michael Beckelic: Braintree Laboratories, Inc. – Employee.
Jack DiPalma, MD1, Raj Bhandari, MD2, Douglas Rex, MD3, Andrew Swanson, 4, Michael E.. Beckelic, BSN5. P0427 - A <i>Post Hoc</i> Safety Analysis of Flavored Polyethylene Glycol and Sulfate Solution Bowel Preparation in Elderly Patients and Patients With Renal Insufficiency, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
