Sunday Poster Session
Category: Colorectal Cancer Prevention
P0428 - Use of Flavored Polyethylene Glycol and Sulfate Solution Bowel Preparation in Patients with Obesity, Diabetes, or Taking Glucagon-Like Peptide-1 Receptor Agonists
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
.jpg)
Michael E. Beckelic, BSN
Braintree Laboratories, Inc.
Roswell, GA
Presenting Author(s)
Eric J. Vargas, MD, MS1, Daniel Pambianco, MD2, Douglas Rex, MD3, Michael E.. Beckelic, BSN4
1Mayo Clinic, Rochester, MN; 2Gastro Health, Charlottesville, VA; 3Indiana University School of Medicine, Indianapolis, IN; 4Braintree Laboratories, Inc., Roswell, GA
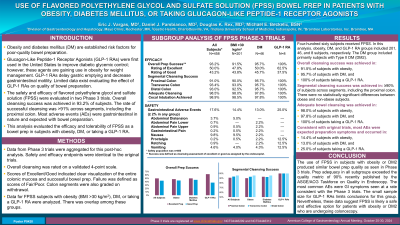
Introduction: Obesity and diabetes mellitus (DM) are risk factors for poor-quality bowel prep. Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RAs) were first used the U.S. to improve diabetic glycemic control; however, are gaining use in obesity for weight management. GLP-1 RAs delay gastric emptying and decrease gastrointestinal motility. Limited data exist evaluating the effect of GLP-1 RAs on bowel prep quality. A recent publication suggested GLP-1 RAs may be a factor in suboptimal bowel prep.
Safety and efficacy of FPSS were evaluated in two Phase 3 trials. Overall cleansing success was achieved in 93.2% of subjects. The rate of successful cleansing was >91% across segments, including the proximal colon. As expected, most adverse events (AEs) were gastrointestinal in nature. This analysis evaluated the efficacy and tolerability of FPSS as a bowel prep in subjects with obesity, DM, or taking a GLP-1 RA.
Methods: Data from Phase 3 trials were aggregated for this post-hoc analysis. Endpoints were identical to the original trials. Overall cleansing was rated on a validated 4-point scale. Scores of Excellent/Good indicated clear visualization of the entire colonic mucosa and successful bowel prep. Failure was defined as scores of Fair/Poor. Colon segments were also graded on withdrawal. Data for FPSS subjects with obesity (BMI >30 kg/m2), DM, or taking a GLP-1 RA were analyzed. There was overlap among these groups.
Results: Four-hundred sixty subjects received FPSS. In this analysis, obesity, DM, and GLP-1 RA groups included 201, 46, and 8 subjects, respectively. Overall cleansing success was achieved in 91.5%, 95.7%, and 100% of subjects with obesity, DM, and taking a GLP-1 RA, respectively. Segmental cleansing success was achieved in ≥90% across segments, including the proximal colon. There were no statistically significant differences between obese and non-obese subjects. Consistent with original trials, most AEs were gastrointestinal and occurred in 14.4%, 13.0%, and 25.0% of FPSS subjects with obesity, DM, or taking a GLP-1 RA, respectively, as presented in Table 1.
Discussion: The use of FPSS in subjects with obesity or DM produced similar bowel prep quality as seen in Phase 3 trials. The most common AEs were gastrointestinal at a rate consistent with the Phase 3 trials. The small sample size for GLP-1 RAs limits conclusions for this group. Nevertheless, these data suggest FPSS may be a safe and effective option for patients with obesity or DM who are undergoing colonoscopy.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Eric J. Vargas, MD, MS1, Daniel Pambianco, MD2, Douglas Rex, MD3, Michael E.. Beckelic, BSN4. P0428 - Use of Flavored Polyethylene Glycol and Sulfate Solution Bowel Preparation in Patients with Obesity, Diabetes, or Taking Glucagon-Like Peptide-1 Receptor Agonists, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2Gastro Health, Charlottesville, VA; 3Indiana University School of Medicine, Indianapolis, IN; 4Braintree Laboratories, Inc., Roswell, GA
Introduction: Obesity and diabetes mellitus (DM) are risk factors for poor-quality bowel prep. Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RAs) were first used the U.S. to improve diabetic glycemic control; however, are gaining use in obesity for weight management. GLP-1 RAs delay gastric emptying and decrease gastrointestinal motility. Limited data exist evaluating the effect of GLP-1 RAs on bowel prep quality. A recent publication suggested GLP-1 RAs may be a factor in suboptimal bowel prep.
Safety and efficacy of FPSS were evaluated in two Phase 3 trials. Overall cleansing success was achieved in 93.2% of subjects. The rate of successful cleansing was >91% across segments, including the proximal colon. As expected, most adverse events (AEs) were gastrointestinal in nature. This analysis evaluated the efficacy and tolerability of FPSS as a bowel prep in subjects with obesity, DM, or taking a GLP-1 RA.
Methods: Data from Phase 3 trials were aggregated for this post-hoc analysis. Endpoints were identical to the original trials. Overall cleansing was rated on a validated 4-point scale. Scores of Excellent/Good indicated clear visualization of the entire colonic mucosa and successful bowel prep. Failure was defined as scores of Fair/Poor. Colon segments were also graded on withdrawal. Data for FPSS subjects with obesity (BMI >30 kg/m2), DM, or taking a GLP-1 RA were analyzed. There was overlap among these groups.
Results: Four-hundred sixty subjects received FPSS. In this analysis, obesity, DM, and GLP-1 RA groups included 201, 46, and 8 subjects, respectively. Overall cleansing success was achieved in 91.5%, 95.7%, and 100% of subjects with obesity, DM, and taking a GLP-1 RA, respectively. Segmental cleansing success was achieved in ≥90% across segments, including the proximal colon. There were no statistically significant differences between obese and non-obese subjects. Consistent with original trials, most AEs were gastrointestinal and occurred in 14.4%, 13.0%, and 25.0% of FPSS subjects with obesity, DM, or taking a GLP-1 RA, respectively, as presented in Table 1.
Discussion: The use of FPSS in subjects with obesity or DM produced similar bowel prep quality as seen in Phase 3 trials. The most common AEs were gastrointestinal at a rate consistent with the Phase 3 trials. The small sample size for GLP-1 RAs limits conclusions for this group. Nevertheless, these data suggest FPSS may be a safe and effective option for patients with obesity or DM who are undergoing colonoscopy.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Eric Vargas: Philips – Grant/Research Support.
Daniel Pambianco indicated no relevant financial relationships.
Douglas Rex: Acacia Pharmaceuticals – Consultant. Boston Scientific – Consultant. Braintree Laboratories – Consultant, Grant/Research Support. Erbe USA – Grant/Research Support. Medivators – Grant/Research Support. Medtronic – Consultant. Norgine – Consultant. Olympus Corporation – Consultant, Grant/Research Support. Satisfai Health – Stock Options.
Michael Beckelic: Braintree Laboratories, Inc. – Employee.
Eric J. Vargas, MD, MS1, Daniel Pambianco, MD2, Douglas Rex, MD3, Michael E.. Beckelic, BSN4. P0428 - Use of Flavored Polyethylene Glycol and Sulfate Solution Bowel Preparation in Patients with Obesity, Diabetes, or Taking Glucagon-Like Peptide-1 Receptor Agonists, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
