Sunday Poster Session
Category: Esophagus
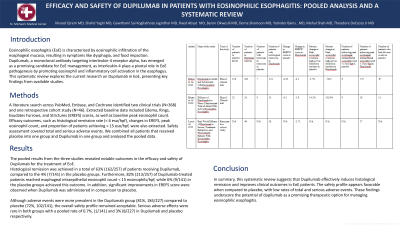
P0502 - Efficacy and Safety of Dupilumab in Patients With Eosinophilic Esophagitis: Pooled Analysis and a Systematic Review
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
- MQ
Murad Qirem, MD
New York Medical College - Saint Michael's Medical Center
Newark, NJ
Presenting Author(s)
Murad Qirem, MD1, Shahd Yaghi, MD1, Gowthami Sai Kogilathota Jagirdhar, MD2, Byron Okwesili, MD1, Raed Atiyat, MD1, Dema Shamoon, MD3, Yatinder Bains, MD1, Mehul Shah, MD1, Theodore DaCosta Jr, MD1
1New York Medical College - Saint Michael's Medical Center, Newark, NJ; 2Saint Michaels Medical Center, Newark, NJ; 3Geisinger Community Medical Center, Danville, PA
Introduction: Eosinophilic esophagitis (EoE) is characterized by eosinophilic infiltration of the esophageal mucosa, resulting in symptoms like dysphagia, and food impaction. Dupilumab, a monoclonal antibody targeting interleukin-4 receptor alpha, has emerged as a promising candidate for EoE management, as Interleukin-4 plays a pivotal role in EoE pathogenesis by promoting eosinophil and inflammatory cell activation in the esophagus.
This systematic review explores the current research on Dupilumab in EoE, presenting key findings from available studies.
Methods: A literature search across PubMed, Embase, and Cochrane identified two clinical trials (N=368) and one retrospective cohort study (N=46). Extracted baseline data included Edema, Rings, Exudates Furrows, and Strictures (EREFS) scores, as well as baseline peak eosinophil count. Efficacy outcomes, such as histological remission rate (< 6 eso/hpf), changes in EREFS, peak eosinophil count, and proportion of patients achieving < 15 eso/hpf, were also extracted. Safety assessment covered total and serious adverse events. We combined all patients that received placebo into one group and Dupilumab in one group and analyzed the pooled data.
Results: The pooled results from the three studies revealed notable outcomes in the efficacy and safety of Dupilumab for the treatment of EoE.
Histological remission was achieved in a total of 63% (162/257) of patients receiving Dupilumab, compared to the 4% (7/141) in the placebo groups. Furthermore, 82% (213/257) of Dupilumab-treated patients reached esophageal intraepithelial eosinophil count < 15 eosinophils/hpf, while 6% (9/141) in the placebo groups achieved this outcome. In addition, significant improvements in EREFS score were observed when Dupilumab was administered in comparison to placebo.
Although adverse events were more prevalent in the Dupilumab group (81%, 184/227) compared to placebo (72%, 102/141), the overall safety profile remained acceptable. Serious adverse effects were rare in both groups with a pooled rate of 0.7%, (1/141) and 3% (8/227) in Dupilumab and placebo respectively.
Discussion: In summary, this systematic review suggests that Dupilumab effectively induces histological remission and improves clinical outcomes in EoE patients. The safety profile appears favorable when compared to placebo, with low rates of total and serious adverse events. These findings underscore the potential of dupilumab as a promising therapeutic option for managing eosinophilic esophagitis.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Murad Qirem, MD1, Shahd Yaghi, MD1, Gowthami Sai Kogilathota Jagirdhar, MD2, Byron Okwesili, MD1, Raed Atiyat, MD1, Dema Shamoon, MD3, Yatinder Bains, MD1, Mehul Shah, MD1, Theodore DaCosta Jr, MD1. P0502 - Efficacy and Safety of Dupilumab in Patients With Eosinophilic Esophagitis: Pooled Analysis and a Systematic Review, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1New York Medical College - Saint Michael's Medical Center, Newark, NJ; 2Saint Michaels Medical Center, Newark, NJ; 3Geisinger Community Medical Center, Danville, PA
Introduction: Eosinophilic esophagitis (EoE) is characterized by eosinophilic infiltration of the esophageal mucosa, resulting in symptoms like dysphagia, and food impaction. Dupilumab, a monoclonal antibody targeting interleukin-4 receptor alpha, has emerged as a promising candidate for EoE management, as Interleukin-4 plays a pivotal role in EoE pathogenesis by promoting eosinophil and inflammatory cell activation in the esophagus.
This systematic review explores the current research on Dupilumab in EoE, presenting key findings from available studies.
Methods: A literature search across PubMed, Embase, and Cochrane identified two clinical trials (N=368) and one retrospective cohort study (N=46). Extracted baseline data included Edema, Rings, Exudates Furrows, and Strictures (EREFS) scores, as well as baseline peak eosinophil count. Efficacy outcomes, such as histological remission rate (< 6 eso/hpf), changes in EREFS, peak eosinophil count, and proportion of patients achieving < 15 eso/hpf, were also extracted. Safety assessment covered total and serious adverse events. We combined all patients that received placebo into one group and Dupilumab in one group and analyzed the pooled data.
Results: The pooled results from the three studies revealed notable outcomes in the efficacy and safety of Dupilumab for the treatment of EoE.
Histological remission was achieved in a total of 63% (162/257) of patients receiving Dupilumab, compared to the 4% (7/141) in the placebo groups. Furthermore, 82% (213/257) of Dupilumab-treated patients reached esophageal intraepithelial eosinophil count < 15 eosinophils/hpf, while 6% (9/141) in the placebo groups achieved this outcome. In addition, significant improvements in EREFS score were observed when Dupilumab was administered in comparison to placebo.
Although adverse events were more prevalent in the Dupilumab group (81%, 184/227) compared to placebo (72%, 102/141), the overall safety profile remained acceptable. Serious adverse effects were rare in both groups with a pooled rate of 0.7%, (1/141) and 3% (8/227) in Dupilumab and placebo respectively.
Discussion: In summary, this systematic review suggests that Dupilumab effectively induces histological remission and improves clinical outcomes in EoE patients. The safety profile appears favorable when compared to placebo, with low rates of total and serious adverse events. These findings underscore the potential of dupilumab as a promising therapeutic option for managing eosinophilic esophagitis.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Murad Qirem indicated no relevant financial relationships.
Shahd Yaghi indicated no relevant financial relationships.
Gowthami Sai Kogilathota Jagirdhar indicated no relevant financial relationships.
Byron Okwesili indicated no relevant financial relationships.
Raed Atiyat indicated no relevant financial relationships.
Dema Shamoon indicated no relevant financial relationships.
Yatinder Bains indicated no relevant financial relationships.
Mehul Shah indicated no relevant financial relationships.
Theodore DaCosta Jr indicated no relevant financial relationships.
Murad Qirem, MD1, Shahd Yaghi, MD1, Gowthami Sai Kogilathota Jagirdhar, MD2, Byron Okwesili, MD1, Raed Atiyat, MD1, Dema Shamoon, MD3, Yatinder Bains, MD1, Mehul Shah, MD1, Theodore DaCosta Jr, MD1. P0502 - Efficacy and Safety of Dupilumab in Patients With Eosinophilic Esophagitis: Pooled Analysis and a Systematic Review, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
