Sunday Poster Session
Category: Functional Bowel Disease
P0642 - Use of Haloperidol in Gastroparesis: A Systemic Review and Meta-Analysis
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio

Sagar Nagpal, MD
East Tennessee State University Quillen College of Medicine
Johnson City, TN
Presenting Author(s)
Sagar Nagpal, MD1, Hezborn Magacha, MD, MPH1, Usama Abu-Heija, MD2, Venkata Vedantam, MD2
1East Tennessee State University Quillen College of Medicine, Johnson City, TN; 2East Tennessee State University, Johnson City, TN
Introduction: Gastroparesis, also known as delayed gastric emptying, refers to a condition where the stomach fails to efficiently move its contents into the small intestine within a normal timeframe. This disorder manifests with symptoms such as recurring nausea, premature feelings of fullness after meals, abdominal bloating, and unintended weight loss. About 200,000 emergency room visits yearly are due to gastroparesis, with approximately 80% of these cases resulting in hospital admissions. We conducted this study to assess the use of haloperidol in the emergency room (ER) for treating gastroparesis. Haloperidol's antiemetic properties stem from its antagonism of dopamine, histamine, and acetylcholine receptors, mitigating nausea and vomiting.
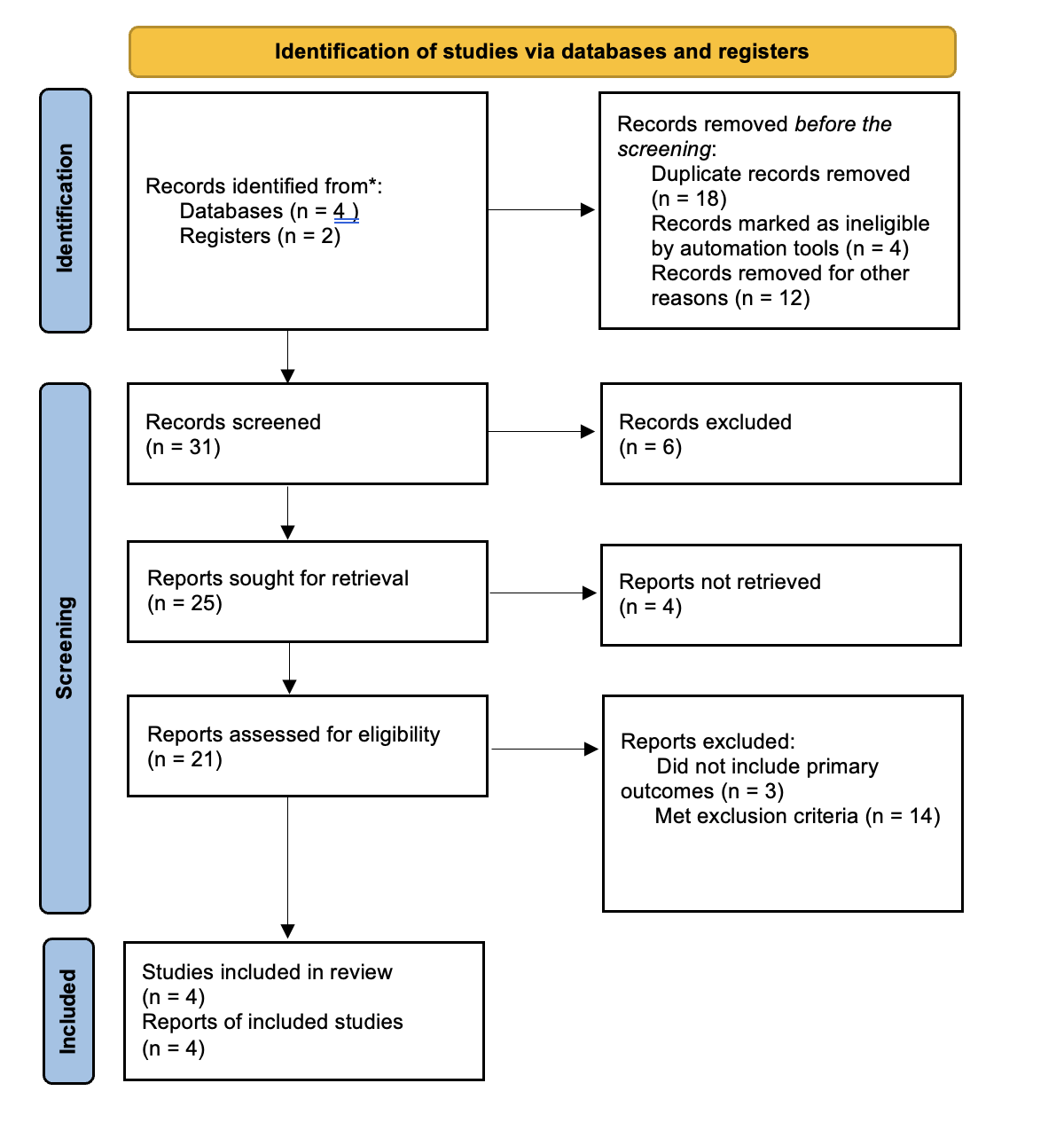
Methods: We conducted a systematic search of databases including Cochrane, Embase, ClinicalTrials.gov, Google Scholar, and Cochrane Clinical Trials, Propero from 2005 to 2025. Two authors independently screened studies evaluating haloperidol's use in gastroparesis patients. Primary outcome of the study was the number of hospitalizations in patients who received haloperidol during ER visit. Inclusion criteria comprised studies comparing haloperidol to conventional treatment without haloperidol, reporting primary outcomes. Exclusion criteria included case reports, review articles, abstracts, and posters. A total of 66 studies were identified, and after rigorous review, four studies met the inclusion criteria. 377 patient encounters were included in the study.
Results: A meta-analysis was conducted to assess the impact of haloperidol on hospital admissions for gastroparesis. The pooled analysis of relevant studies revealed a statistically significant reduction in hospital admissions among patients treated with haloperidol compared to those who received placebo or standard care (Risk Ratio = 0.55, 95% CI 0.34 to 0.88, p = 0.02).
This finding suggests that haloperidol administration is associated with a 45% decreased relative risk of hospital admission for gastroparesis patients, highlighting its potential efficacy in managing these patients.
Discussion: Haloperidol shows promise as a therapeutic intervention to mitigate the need for hospitalization in gastroparesis patients. Further well-designed clinical trials are warranted to validate these findings and elucidate the optimal dosing and timing of haloperidol administration in this context.
Disclosures:
Sagar Nagpal, MD1, Hezborn Magacha, MD, MPH1, Usama Abu-Heija, MD2, Venkata Vedantam, MD2. P0642 - Use of Haloperidol in Gastroparesis: A Systemic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1East Tennessee State University Quillen College of Medicine, Johnson City, TN; 2East Tennessee State University, Johnson City, TN
Introduction: Gastroparesis, also known as delayed gastric emptying, refers to a condition where the stomach fails to efficiently move its contents into the small intestine within a normal timeframe. This disorder manifests with symptoms such as recurring nausea, premature feelings of fullness after meals, abdominal bloating, and unintended weight loss. About 200,000 emergency room visits yearly are due to gastroparesis, with approximately 80% of these cases resulting in hospital admissions. We conducted this study to assess the use of haloperidol in the emergency room (ER) for treating gastroparesis. Haloperidol's antiemetic properties stem from its antagonism of dopamine, histamine, and acetylcholine receptors, mitigating nausea and vomiting.
Methods: We conducted a systematic search of databases including Cochrane, Embase, ClinicalTrials.gov, Google Scholar, and Cochrane Clinical Trials, Propero from 2005 to 2025. Two authors independently screened studies evaluating haloperidol's use in gastroparesis patients. Primary outcome of the study was the number of hospitalizations in patients who received haloperidol during ER visit. Inclusion criteria comprised studies comparing haloperidol to conventional treatment without haloperidol, reporting primary outcomes. Exclusion criteria included case reports, review articles, abstracts, and posters. A total of 66 studies were identified, and after rigorous review, four studies met the inclusion criteria. 377 patient encounters were included in the study.
Results: A meta-analysis was conducted to assess the impact of haloperidol on hospital admissions for gastroparesis. The pooled analysis of relevant studies revealed a statistically significant reduction in hospital admissions among patients treated with haloperidol compared to those who received placebo or standard care (Risk Ratio = 0.55, 95% CI 0.34 to 0.88, p = 0.02).
This finding suggests that haloperidol administration is associated with a 45% decreased relative risk of hospital admission for gastroparesis patients, highlighting its potential efficacy in managing these patients.
Discussion: Haloperidol shows promise as a therapeutic intervention to mitigate the need for hospitalization in gastroparesis patients. Further well-designed clinical trials are warranted to validate these findings and elucidate the optimal dosing and timing of haloperidol administration in this context.

Figure: PRISMA flow diagram illustrating the search strategy for studies included in the systematic review.
Disclosures:
Sagar Nagpal indicated no relevant financial relationships.
Hezborn Magacha indicated no relevant financial relationships.
Usama Abu-Heija indicated no relevant financial relationships.
Venkata Vedantam indicated no relevant financial relationships.
Sagar Nagpal, MD1, Hezborn Magacha, MD, MPH1, Usama Abu-Heija, MD2, Venkata Vedantam, MD2. P0642 - Use of Haloperidol in Gastroparesis: A Systemic Review and Meta-Analysis, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
