Sunday Poster Session
Category: IBD
P0825 - Integrated Long-Term Safety of Ozanimod From Clinical Trials Across 2 Different Indications
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
- DR
David T. Rubin, MD, FACG
University of Chicago Medicine, Inflammatory Bowel Disease Center
Chicago, IL
Presenting Author(s)
David T.. Rubin, MD, FACG1, Silvio Danese, MD, PhD2, Peter M.. Irving, MA, MD3, Hiroshi Nakase, MD4, Douglas C. Wolf, MD, FACG5, Preetika Sinh, MD6, Bruce A.C. Cree, MD, PhD7, Olga Alekseeva, MD8, Fred D. Lublin, MD9, Norma Ruiz Santiago, MD10, Zhaohui Liu, PhD10, AnnKatrin Petersen, MD10, Dimpy Mehra, PharmD10, Anjali Jain, PhD10, Anthony Krakovich, MPH10, Chun-Yen Cheng, MS10, Jon V. Riolo, PhD10, Erik DeBoer, PhD10, Jeffrey A. Cohen, MD11, Ryan C. Ungaro, MD9
1University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 2Humanitas Clinical and Research Center - IRCCS, Rozzano and Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 3Guy’s and St. Thomas’ NHS Foundation Trust, London, England, United Kingdom; 4Sapporo Medical University, Sapporo, Hokkaido, Japan; 5Center for Crohn’s Disease & Ulcerative Colitis, Atlanta Gastroenterology Associates, Atlanta, GA; 6Medical College of Wisconsin, Milwaukee, WI; 7Weill Institute for Neurosciences, University of California San Francisco, San Francisco, CA; 8Nizhny Novgorod Regional Clinical Hospital, Nizhny Novgorod, Novgorod, Russia; 9Icahn School of Medicine at Mount Sinai, New York, NY; 10Bristol Myers Squibb, Princeton, NJ; 11Mellen Center for Multiple Sclerosis Treatment and Research, Cleveland Clinic, Cleveland, OH
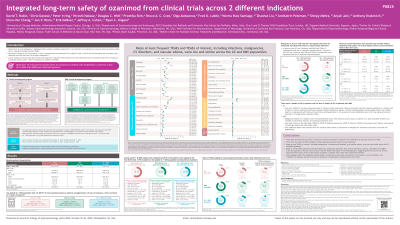
Introduction: Ozanimod (OZA) is a highly selective sphingosine 1-phosphate (S1P) receptor 1 and 5 modulator approved for the treatment (tx) of moderately to severely active ulcerative colitis (UC) or relapsing multiple sclerosis (RMS). A prior analysis of clinical trial data showed that long-term tx with OZA was well tolerated across 2715 patient-years (PY) of exposure in patients (pts) with UC and 11,732 PY of exposure in pts with RMS. This analysis provides a pooled safety evaluation of OZA in pts with moderately to severely active UC or RMS with additional OZA exposure.
Methods: Pooled data in pts with UC who received OZA in phase 2 (NCT01647516), phase 3 (NCT02435592), and respective open-label extension (OLE; NCT02531126) trials were examined from December 2, 2015, through November 17, 2023. Data in pts with RMS who received OZA in an OLE trial (NCT02576717) after enrolling from phase 1–3 trials were examined from October 16, 2015, through April 7, 2023. Outcomes included treatment-emergent adverse events (TEAEs) and laboratory abnormalities.
Results: Total OZA exposure was 3479 PY in 1158 pts with UC and 12,663 PY in 2494 pts with RMS (16,142 PY in 3652 pts total). TEAEs, serious TEAEs, TEAEs leading to tx discontinuation, and TEAEs of interest in the UC, RMS, and combined UC + RMS populations are reported in the Table. Exposure-adjusted incidence rates (EAIRs) of most TEAEs of interest were low and similar across populations. The most common TEAEs as reported by investigators were lymphopenia (13.0%, EAIR 48.8/1000 PY) and COVID-19 (10.2%, EAIR 36.3/1000 PY) in pts with UC and nasopharyngitis (21.3%, EAIR 49.6/1000 PY) and headache (17.1%, EAIR 38.3/1000 PY) in pts with RMS. Absolute lymphocyte count < 200 cells/µl occurred in 6.8% of pts with UC and 14.8% of pts with RMS during OLE trials but were not temporally associated with serious or opportunistic infections. Most liver enzyme elevations during OLE trials were transient and resolved without tx interruption; no serious hepatic events or Hy’s law cases occurred. There were 5 deaths (0.4%) in UC pts and 15 deaths (0.6%) in RMS pts.
Discussion: Long-term exposure to OZA evaluated over 16,000 PYs in clinical trials continues to be well tolerated in pts with moderately to severely active UC or RMS. These data confirm the established safety profile of OZA, a once-daily oral advanced therapy, with high selectivity for S1P receptors 1 and 5.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
David T.. Rubin, MD, FACG1, Silvio Danese, MD, PhD2, Peter M.. Irving, MA, MD3, Hiroshi Nakase, MD4, Douglas C. Wolf, MD, FACG5, Preetika Sinh, MD6, Bruce A.C. Cree, MD, PhD7, Olga Alekseeva, MD8, Fred D. Lublin, MD9, Norma Ruiz Santiago, MD10, Zhaohui Liu, PhD10, AnnKatrin Petersen, MD10, Dimpy Mehra, PharmD10, Anjali Jain, PhD10, Anthony Krakovich, MPH10, Chun-Yen Cheng, MS10, Jon V. Riolo, PhD10, Erik DeBoer, PhD10, Jeffrey A. Cohen, MD11, Ryan C. Ungaro, MD9. P0825 - Integrated Long-Term Safety of Ozanimod From Clinical Trials Across 2 Different Indications, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 2Humanitas Clinical and Research Center - IRCCS, Rozzano and Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 3Guy’s and St. Thomas’ NHS Foundation Trust, London, England, United Kingdom; 4Sapporo Medical University, Sapporo, Hokkaido, Japan; 5Center for Crohn’s Disease & Ulcerative Colitis, Atlanta Gastroenterology Associates, Atlanta, GA; 6Medical College of Wisconsin, Milwaukee, WI; 7Weill Institute for Neurosciences, University of California San Francisco, San Francisco, CA; 8Nizhny Novgorod Regional Clinical Hospital, Nizhny Novgorod, Novgorod, Russia; 9Icahn School of Medicine at Mount Sinai, New York, NY; 10Bristol Myers Squibb, Princeton, NJ; 11Mellen Center for Multiple Sclerosis Treatment and Research, Cleveland Clinic, Cleveland, OH
Introduction: Ozanimod (OZA) is a highly selective sphingosine 1-phosphate (S1P) receptor 1 and 5 modulator approved for the treatment (tx) of moderately to severely active ulcerative colitis (UC) or relapsing multiple sclerosis (RMS). A prior analysis of clinical trial data showed that long-term tx with OZA was well tolerated across 2715 patient-years (PY) of exposure in patients (pts) with UC and 11,732 PY of exposure in pts with RMS. This analysis provides a pooled safety evaluation of OZA in pts with moderately to severely active UC or RMS with additional OZA exposure.
Methods: Pooled data in pts with UC who received OZA in phase 2 (NCT01647516), phase 3 (NCT02435592), and respective open-label extension (OLE; NCT02531126) trials were examined from December 2, 2015, through November 17, 2023. Data in pts with RMS who received OZA in an OLE trial (NCT02576717) after enrolling from phase 1–3 trials were examined from October 16, 2015, through April 7, 2023. Outcomes included treatment-emergent adverse events (TEAEs) and laboratory abnormalities.
Results: Total OZA exposure was 3479 PY in 1158 pts with UC and 12,663 PY in 2494 pts with RMS (16,142 PY in 3652 pts total). TEAEs, serious TEAEs, TEAEs leading to tx discontinuation, and TEAEs of interest in the UC, RMS, and combined UC + RMS populations are reported in the Table. Exposure-adjusted incidence rates (EAIRs) of most TEAEs of interest were low and similar across populations. The most common TEAEs as reported by investigators were lymphopenia (13.0%, EAIR 48.8/1000 PY) and COVID-19 (10.2%, EAIR 36.3/1000 PY) in pts with UC and nasopharyngitis (21.3%, EAIR 49.6/1000 PY) and headache (17.1%, EAIR 38.3/1000 PY) in pts with RMS. Absolute lymphocyte count < 200 cells/µl occurred in 6.8% of pts with UC and 14.8% of pts with RMS during OLE trials but were not temporally associated with serious or opportunistic infections. Most liver enzyme elevations during OLE trials were transient and resolved without tx interruption; no serious hepatic events or Hy’s law cases occurred. There were 5 deaths (0.4%) in UC pts and 15 deaths (0.6%) in RMS pts.
Discussion: Long-term exposure to OZA evaluated over 16,000 PYs in clinical trials continues to be well tolerated in pts with moderately to severely active UC or RMS. These data confirm the established safety profile of OZA, a once-daily oral advanced therapy, with high selectivity for S1P receptors 1 and 5.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
David Rubin: AbbVie – Consultant. AltruBio – Consultant. Apex – Consultant. Avalo Therapeutics – Consultant. Bausch Health – Consultant. Bristol Myers Squibb – Consultant. Buhlmann Diagnostics Corp – Consultant. Celgene – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health – Board of Directors. Crohn's & Colitis Foundation – Board of Trustees. Douglas Therapeutics – Consultant. Eli Lilly – Consultant. InDex Pharmaceuticals – Consultant. Intouch Group – Consultant. Iterative Health – Consultant. Janssen Pharmaceuticals – Consultant. Odyssey Thera – Consultant. Pfizer – Consultant. Prometheus Biosciences – Consultant. Samsung Neurologica – Consultant. Takeda – Consultant, Grant/Research Support.
Silvio Danese: AbbVie – Consultant, Speakers Bureau. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Speakers Bureau. Applied Molecular Transport – Consultant. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Celltrion Healthcare – Consultant. Dr Falk Pharma – Consultant. Eli Lilly and Company – Consultant. Enthera – Consultant. Ferring – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. Hospira – Consultant. Inotrem – Consultant. Janssen – Consultant, Speakers Bureau. Johnson & Johnson – Consultant. Morphic – Consultant. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Speakers Bureau. Roche – Consultant. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Speakers Bureau. Teladoc Health – Consultant. TiGenix – Consultant. UCB Inc. – Consultant. Vial – Consultant. Vifor – Consultant.
Peter Irving: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Arena – Advisory Committee/Board Member. Boehringer Ingelheim – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celgene – Advisory Committee/Board Member, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Dr. Falk Pharma – Speakers Bureau. Eli Lilly – Advisory Committee/Board Member. Ferring – Speakers Bureau. Galapagos – Speakers Bureau. Genentech – Advisory Committee/Board Member. Gilead – Advisory Committee/Board Member, Speakers Bureau. Hospira – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Speakers Bureau. MSD – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pharmacosmos – Advisory Committee/Board Member. Prometheus Biosciences – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member. Samsung Bioepis – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member, Speakers Bureau. Sapphire Medical – Speakers Bureau. Shire – Speakers Bureau. Takeda – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Tillotts – Speakers Bureau. TopiVert – Advisory Committee/Board Member. VH2 – Advisory Committee/Board Member. Vifor – Advisory Committee/Board Member. Warner Chilcott – Advisory Committee/Board Member, Speakers Bureau.
Hiroshi Nakase: AbbVie GK – Grant/Research Support, Speakers Bureau. Astellas – Speakers Bureau. AYUMI Pharmaceutical – Grant/Research Support. EA Pharma – Speakers Bureau. Gilead Sciences – Speakers Bureau. HOYA Pentax Medical – Grant/Research Support. Janssen – Speakers Bureau. KYORIN – Speakers Bureau. Mitsubishi Tanabe – Grant/Research Support, Speakers Bureau. Mochida – Grant/Research Support, Speakers Bureau. Nippon Kayaku – Speakers Bureau. Pfizer – Speakers Bureau. Takeda – Speakers Bureau. Viatris – Speakers Bureau. Zeria Pharmaceutical – Speakers Bureau.
Douglas C. Wolf: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Amgen – Grant/Research Support. Arena/Pfizer – Consultant, Grant/Research Support. Celgene/Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly – Consultant. Genentech – Grant/Research Support. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Takeda – Consultant, Speakers Bureau.
Preetika Sinh: Bristol Myers Squibb – Advisory Committee/Board Member.
Bruce A.C. Cree: Alexion – Consultant. Atara – Consultant. Autobahn – Consultant. Avotres – Consultant. Biogen – Consultant. Boston Pharma – Consultant. EMD Serono – Consultant. Genentech – Grant/Research Support. Gossamer Bio – Consultant. Hexal/Sandoz – Consultant. Horizon – Consultant. Immunic AG – Consultant. Kyverna – Consultant. Neuron 23 – Consultant. Novartis – Consultant. Sanofi – Consultant. Siemens – Consultant. TG Therapeutics – Consultant.
Olga Alekseeva: Janssen – Speakers Bureau. Pfizer – Speakers Bureau. Takeda – Speakers Bureau.
Fred D. Lublin: Acorda – Advisory Committee/Board Member, Consultant, DSMBs. Actelion – Advisory Committee/Board Member, Consultant, DSMBs. Apitope – Advisory Committee/Board Member, Consultant, DSMBs. Atara Biotherapeutics – Advisory Committee/Board Member, Consultant, DSMBs. Biogen – Advisory Committee/Board Member, Consultant, DSMBs. Brainstorm Cell Therapeutics – Advisory Committee/Board Member, Consultant, DSMBs. EMD Serono – Advisory Committee/Board Member, Consultant, DSMBs. GW Pharma – Advisory Committee/Board Member, Consultant, DSMBs. Immunic – Advisory Committee/Board Member, Consultant, DSMBs. Innate Immunotherapeutics – Advisory Committee/Board Member, Consultant, DSMBs. Jazz Pharmaceuticals – Advisory Committee/Board Member, Consultant, DSMBs. Mapi Pharma – Advisory Committee/Board Member, Consultant, DSMBs. MedDay – Advisory Committee/Board Member, Consultant, DSMBs. MedImmune/Viela Bio – Advisory Committee/Board Member, Consultant, DSMBs. Mylan – Advisory Committee/Board Member, Consultant, DSMBs. Novartis – Advisory Committee/Board Member, Consultant, DSMBs. Orion Biotechnology – Advisory Committee/Board Member, Consultant, DSMBs. Polpharma – Advisory Committee/Board Member, Consultant, DSMBs. Population Council – Advisory Committee/Board Member, Consultant, DSMBs. Receptos/Celgene – Advisory Committee/Board Member, Consultant, DSMBs. Roche/Genentech – Advisory Committee/Board Member, Consultant, DSMBs. Sanofi/Genzyme – Advisory Committee/Board Member, Consultant, DSMBs. Teva – Advisory Committee/Board Member, Consultant, DSMBs. TG Therapeutics – Advisory Committee/Board Member, Consultant, DSMBs.
Norma Ruiz Santiago: Bristol Myers Squibb – Employee.
Zhaohui Liu: Bristol Myers Squibb – Employee.
AnnKatrin Petersen: Bristol Myers Squibb – Employee.
Dimpy Mehra: Bristol Myers Squibb – Employee.
Anjali Jain: Bristol Myers Squibb – Employee.
Anthony Krakovich: Bristol Myers Squibb – Employee.
Chun-Yen Cheng: Bristol Myers Squibb – Employee.
Jon V. Riolo: Bristol Myers Squibb – Employee.
Erik DeBoer: Bristol Myers Squibb – Employee.
Jeffrey A. Cohen: Astoria – Consultant. Bristol Myers Squibb – Consultant. Convelo – Consultant. EMD Serono – Consultant. Find Therapeutics – Consultant. INmune – Consultant. Multiple Sclerosis Journal – editor. Sandoz – Consultant.
Ryan C. Ungaro: AbbVie – Advisory Committee/Board Member, Grant/Research Support. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member, Consultant. Eli Lilly – Grant/Research Support. Inotrem – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Grant/Research Support. Prometheus Labratories – Grant/Research Support. Roivant – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member.
David T.. Rubin, MD, FACG1, Silvio Danese, MD, PhD2, Peter M.. Irving, MA, MD3, Hiroshi Nakase, MD4, Douglas C. Wolf, MD, FACG5, Preetika Sinh, MD6, Bruce A.C. Cree, MD, PhD7, Olga Alekseeva, MD8, Fred D. Lublin, MD9, Norma Ruiz Santiago, MD10, Zhaohui Liu, PhD10, AnnKatrin Petersen, MD10, Dimpy Mehra, PharmD10, Anjali Jain, PhD10, Anthony Krakovich, MPH10, Chun-Yen Cheng, MS10, Jon V. Riolo, PhD10, Erik DeBoer, PhD10, Jeffrey A. Cohen, MD11, Ryan C. Ungaro, MD9. P0825 - Integrated Long-Term Safety of Ozanimod From Clinical Trials Across 2 Different Indications, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
