Sunday Poster Session
Category: IBD
P0850 - Effect of Upadacitinib Treatment on Hematological Parameters in Patients With Moderate to Severe Active Crohn’s Disease or Ulcerative Colitis: Pooled Analysis of Phase 3 Induction and Maintenance Studies
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio

Marla Dubinsky, MD
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Marla Dubinsky, MD1, Miguel D Regueiro, MD2, Christina Ha, MD3, Gil Y. Melmed, MD4, Alessandro Armuzzi, MD, PhD5, Peter M.. Irving, MA, MD6, Samuel Anyanwu, MD7, Elena Dubcenco, MD7, Fernando Aponte, MD7, Isheeta Shah, MD7, Tao Wang, MD7, Smitha Suravaram, MD8, Hannah Palac, MD7, David T.. Rubin, MD, FACG9
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Cleveland Clinic, Cleveland, OH; 3Mayo Clinic Arizona, North Chicago, IL; 4Cedars-Sinai Medical Center, Los Angeles, CA; 5IBD Unit, IRCCS Humanitas Research Hospital, Rozzano, Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 6Guy’s and St. Thomas’ NHS Foundation Trust, London, England, United Kingdom; 7AbbVie, North Chicago, IL; 8AbbVie Inc., North Chicago, IL; 9University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL
Introduction: Upadacitinib (UPA) is an oral Janus kinase inhibitor approved for the treatment of moderate-to-severe Crohn’s disease (CD) and ulcerative colitis (UC). Patients with CD and UC often experience changes in hematological parameters. However, data on hematological changes in UPA-treated patients with CD and UC are limited.
Methods: This post hoc analysis evaluated changes in hematological parameters over time in phase 3 trials of UPA in CD (U-EXCEL, U-EXCEED, U-ENDURE) and UC (U-ACCOMPLISH, U-ACHIEVE) (Loftus EV, Jr., et al. NEJM 2023; Danese et al. Lancet 2022). Data from UC and CD induction and maintenance studies were pooled. Least-square mean (LSM) changes from baseline (BL) with standard errors (SE), the proportions of patients with Grade 1-4 reductions in hematologic parameters (hemoglobin, platelets, leukocytes, neutrophils, and lymphocytes), and the proportions of patients with Grade 1-4 neutropenia or lymphopenia reductions associated with infections or serious infections were quantified.
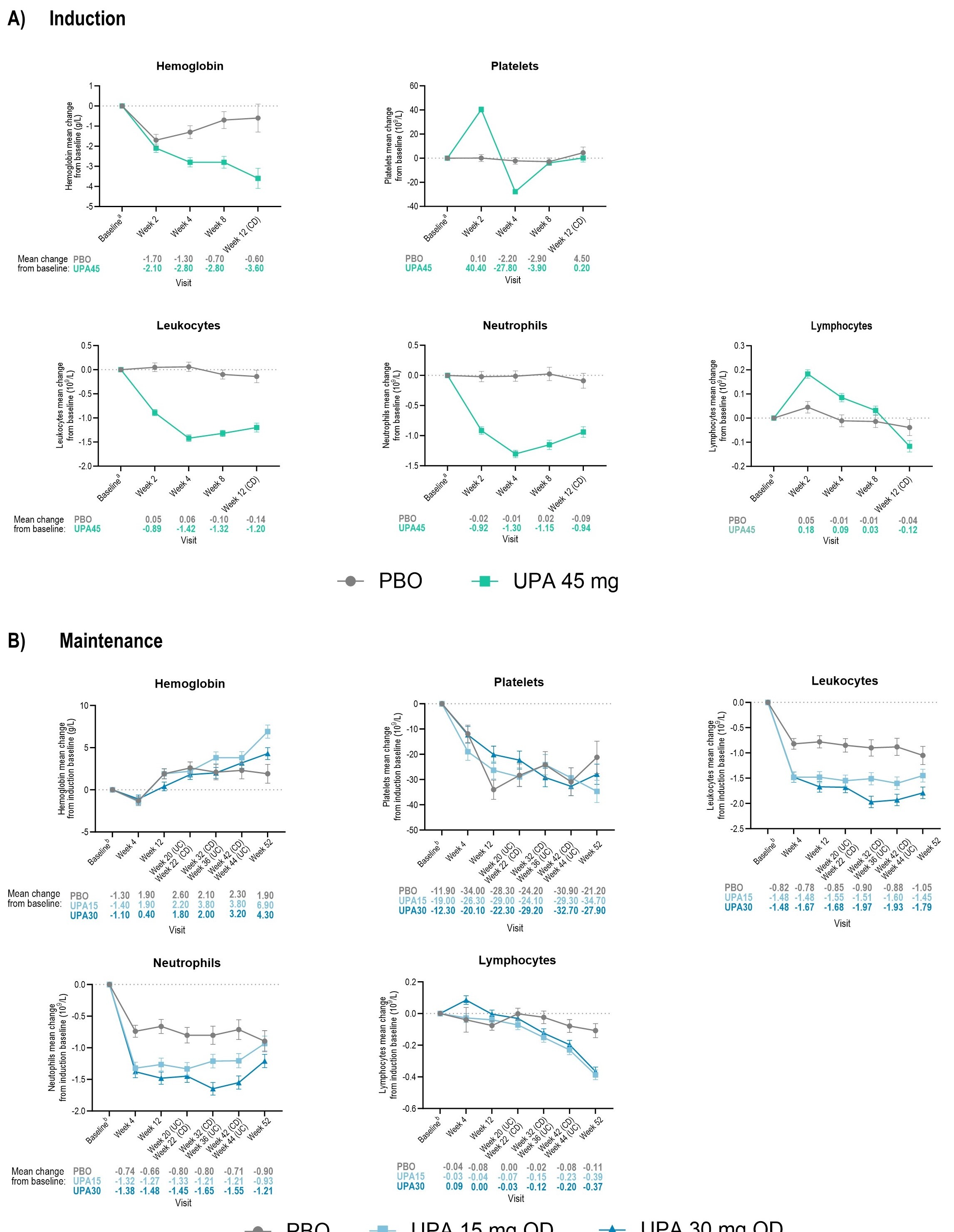
Results: At induction BL, placebo (PBO) and UPA 45 mg (UPA45) mean [SD] hemoglobin (130.1 [18.1] g/L; 129.6 [17.4] g/L), platelets (353.7 [125.8] 109/L; 351.8 [116.4] 109/L), leukocytes (8.5 [3.1] 109/L; 8.4 [2.9] 109/L), neutrophils (6.0 [2.7] 109/L; 5.9 [2.6] 109/L), and lymphocytes (1.8 [0.8] 109/L;1.8 [0.8] 109/L), were similar across treatment arms. LSM [SE] changes from BL in UPA-treated patients for platelets, leukocytes, and neutrophils, were -27.8 [2.0] 109/L, -1.4 [0.1] 109/L, -1.3 [0.1] 109/L, respectively, by induction wk4 and remained relatively stable across all treatment groups through maintenance wk52, except for lymphocytes and hemoglobin; transient modifications were observed in both lymphocyte and hemoglobin levels through induction and maintenance. Infection and serious infection rates were low among patients with Grade 3-4 neutropenia (< 1.0 109/L) or lymphopenia (< 0.5 109/L) (Table 1). Discontinuation due to hematological changes was low across treatment groups during induction and maintenance (0.1% and 0.4% per parameter, respectively).
Discussion: Most hematological changes among UPA-treated patients were mild/moderate, with effects appearing to be transient and few (< 1%) leading to drug interruptions due to Grade 3-4 neutropenia/lymphopenia and associated infections. The effect of hematological parameters should be evaluated in the context of the overall benefit-risk profile.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Marla Dubinsky, MD1, Miguel D Regueiro, MD2, Christina Ha, MD3, Gil Y. Melmed, MD4, Alessandro Armuzzi, MD, PhD5, Peter M.. Irving, MA, MD6, Samuel Anyanwu, MD7, Elena Dubcenco, MD7, Fernando Aponte, MD7, Isheeta Shah, MD7, Tao Wang, MD7, Smitha Suravaram, MD8, Hannah Palac, MD7, David T.. Rubin, MD, FACG9. P0850 - Effect of Upadacitinib Treatment on Hematological Parameters in Patients With Moderate to Severe Active Crohn’s Disease or Ulcerative Colitis: Pooled Analysis of Phase 3 Induction and Maintenance Studies, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Cleveland Clinic, Cleveland, OH; 3Mayo Clinic Arizona, North Chicago, IL; 4Cedars-Sinai Medical Center, Los Angeles, CA; 5IBD Unit, IRCCS Humanitas Research Hospital, Rozzano, Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 6Guy’s and St. Thomas’ NHS Foundation Trust, London, England, United Kingdom; 7AbbVie, North Chicago, IL; 8AbbVie Inc., North Chicago, IL; 9University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL
Introduction: Upadacitinib (UPA) is an oral Janus kinase inhibitor approved for the treatment of moderate-to-severe Crohn’s disease (CD) and ulcerative colitis (UC). Patients with CD and UC often experience changes in hematological parameters. However, data on hematological changes in UPA-treated patients with CD and UC are limited.
Methods: This post hoc analysis evaluated changes in hematological parameters over time in phase 3 trials of UPA in CD (U-EXCEL, U-EXCEED, U-ENDURE) and UC (U-ACCOMPLISH, U-ACHIEVE) (Loftus EV, Jr., et al. NEJM 2023; Danese et al. Lancet 2022). Data from UC and CD induction and maintenance studies were pooled. Least-square mean (LSM) changes from baseline (BL) with standard errors (SE), the proportions of patients with Grade 1-4 reductions in hematologic parameters (hemoglobin, platelets, leukocytes, neutrophils, and lymphocytes), and the proportions of patients with Grade 1-4 neutropenia or lymphopenia reductions associated with infections or serious infections were quantified.
Results: At induction BL, placebo (PBO) and UPA 45 mg (UPA45) mean [SD] hemoglobin (130.1 [18.1] g/L; 129.6 [17.4] g/L), platelets (353.7 [125.8] 109/L; 351.8 [116.4] 109/L), leukocytes (8.5 [3.1] 109/L; 8.4 [2.9] 109/L), neutrophils (6.0 [2.7] 109/L; 5.9 [2.6] 109/L), and lymphocytes (1.8 [0.8] 109/L;1.8 [0.8] 109/L), were similar across treatment arms. LSM [SE] changes from BL in UPA-treated patients for platelets, leukocytes, and neutrophils, were -27.8 [2.0] 109/L, -1.4 [0.1] 109/L, -1.3 [0.1] 109/L, respectively, by induction wk4 and remained relatively stable across all treatment groups through maintenance wk52, except for lymphocytes and hemoglobin; transient modifications were observed in both lymphocyte and hemoglobin levels through induction and maintenance. Infection and serious infection rates were low among patients with Grade 3-4 neutropenia (< 1.0 109/L) or lymphopenia (< 0.5 109/L) (Table 1). Discontinuation due to hematological changes was low across treatment groups during induction and maintenance (0.1% and 0.4% per parameter, respectively).
Discussion: Most hematological changes among UPA-treated patients were mild/moderate, with effects appearing to be transient and few (< 1%) leading to drug interruptions due to Grade 3-4 neutropenia/lymphopenia and associated infections. The effect of hematological parameters should be evaluated in the context of the overall benefit-risk profile.

Figure: Figure 1. Mean Change From Baseline in Select Hematology Parameters During Induction and Maintenance Studies. Data are presented for patients who have both baseline and post baseline values.
BL (week 0 of induction for induction and maintenance graphs) was defined as the last nonmissing observation before the first dose of the study drug. Within each group, the LSM and SE were based on an ANCOVA model with treatment as the main factor and baseline and indication as covariates.
aAt baseline of induction, the number of patients for each treatment group were as follows: hemoglobin, n = 723 PBO and n = 1386 UPA 45 mg QD; platelets PBO n = 717 and UPA 45 mg QD n = 1381; leukocytes PBO n = 722 and UPA 45 mg QD n = 1386; neutrophils PBO n = 720 and UPA 45 mg QD n = 1386; lymphocytes PBO n = 720 and UPA 45 mg QD n = 1385;
bAt baseline of induction, the number of patients for each treatment group were as follows: hemoglobin, n = 461 PBO and n = 469 UPA 15 mg QD, UPA 30 mg QD n = 479; platelets PBO n = 459, UPA 15 mg QD n = 468, UPA 30 mg QD n = 476; leukocytes PBO n = 461, UPA 15 mg QD n = 469, UPA 30 mg QD n = 479; neutrophils PBO n = 460, UPA 15 mg QD n = 468, UPA 30 mg QD n = 479; and lymphocytes PBO n = 460, UPA 15 mg QD n = 468, UPA 30 mg QD n = 479.
ANCOVA, analysis of covariance; BL, baseline; CI, confidence interval; LSM, least-square mean; PBO, placebo; QD, once daily; SE, standard error; UPA, upadacitinib.
BL (week 0 of induction for induction and maintenance graphs) was defined as the last nonmissing observation before the first dose of the study drug. Within each group, the LSM and SE were based on an ANCOVA model with treatment as the main factor and baseline and indication as covariates.
aAt baseline of induction, the number of patients for each treatment group were as follows: hemoglobin, n = 723 PBO and n = 1386 UPA 45 mg QD; platelets PBO n = 717 and UPA 45 mg QD n = 1381; leukocytes PBO n = 722 and UPA 45 mg QD n = 1386; neutrophils PBO n = 720 and UPA 45 mg QD n = 1386; lymphocytes PBO n = 720 and UPA 45 mg QD n = 1385;
bAt baseline of induction, the number of patients for each treatment group were as follows: hemoglobin, n = 461 PBO and n = 469 UPA 15 mg QD, UPA 30 mg QD n = 479; platelets PBO n = 459, UPA 15 mg QD n = 468, UPA 30 mg QD n = 476; leukocytes PBO n = 461, UPA 15 mg QD n = 469, UPA 30 mg QD n = 479; neutrophils PBO n = 460, UPA 15 mg QD n = 468, UPA 30 mg QD n = 479; and lymphocytes PBO n = 460, UPA 15 mg QD n = 468, UPA 30 mg QD n = 479.
ANCOVA, analysis of covariance; BL, baseline; CI, confidence interval; LSM, least-square mean; PBO, placebo; QD, once daily; SE, standard error; UPA, upadacitinib.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant. BMS – Consultant. Boehringer Ingelheim – Consultant. Celgene Corporation – Consultant. Gilead – Consultant. Janssen – Consultant, Grant/Research Support. Lilly – Consultant. Pfizer – Consultant. Prometheus Labs – Consultant. Sanofi – Consultant. Shire – Consultant. Takeda – Consultant.
Miguel D Regueiro: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Christina Ha: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Bausch Health – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member, Grant/Research Support. Helmsley Charitable trust – Grant/Research Support. Janssen – Advisory Committee/Board Member, Grant/Research Support. Pfizer – Advisory Committee/Board Member, Grant/Research Support. Roivant – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member.
Gil Y. Melmed: AbbVie – Consultant. Arena Biopharmaceuticals – Consultant. Boehringer-Ingelheim – Consultant. Bristol Myers Squibb-Celgene – Consultant. Dieta – Consultant. Eli Lilly – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Genentech – Consultant. Gilead – Consultant. Janssen Immunology – Consultant. Oshi Health – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus Labs – Consultant. Samsung Bioepis – Consultant. Shionogi – Consultant. Takeda – Consultant. Techlab – Consultant. Viatris – Consultant.
Alessandro Armuzzi: AbbVie – Consultant, Speakers Bureau. Allergan – Advisory Committee/Board Member, Consultant. Amgen – Consultant, Speakers Bureau. Arena – Advisory Committee/Board Member, Consultant. Biogen – Consultant, Grant/Research Support, Speakers Bureau. Boehringer Ingleheim – Consultant. Bristol-Myers Squibb – Consultant, Grant/Research Support. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Gilead Sciences – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Lionhealth – Consultant, Speakers Bureau. Mitsubishi Tanabe – Speakers Bureau. MSD – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant. Nestle – Consultant. Novartis – Speakers Bureau. Pfizer Inc – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Speakers Bureau. Samsung Bioepis – Consultant, Speakers Bureau. Sandoz – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. TiGenix – Speakers Bureau. Tillots – Consultant.
Peter Irving: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Arena – Advisory Committee/Board Member. Boehringer Ingelheim – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celgene – Advisory Committee/Board Member, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Dr. Falk Pharma – Speakers Bureau. Eli Lilly – Advisory Committee/Board Member. Ferring – Speakers Bureau. Galapagos – Speakers Bureau. Genentech – Advisory Committee/Board Member. Gilead – Advisory Committee/Board Member, Speakers Bureau. Hospira – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Speakers Bureau. MSD – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pharmacosmos – Advisory Committee/Board Member. Prometheus Biosciences – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member. Samsung Bioepis – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member, Speakers Bureau. Sapphire Medical – Speakers Bureau. Shire – Speakers Bureau. Takeda – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Tillotts – Speakers Bureau. TopiVert – Advisory Committee/Board Member. VH2 – Advisory Committee/Board Member. Vifor – Advisory Committee/Board Member. Warner Chilcott – Advisory Committee/Board Member, Speakers Bureau.
Samuel Anyanwu: AbbVie – Employee, Stock Options.
Elena Dubcenco: AbbVie – Employee, Stock Options.
Fernando Aponte: AbbVie – Employee, Stock Options.
Isheeta Shah: AbbVie – Employee, Stock Options.
Tao Wang: AbbVie – Employee, Stock Options.
Smitha Suravaram: AbbVie – Employee, Stock Options.
Hannah Palac: AbbVie – Employee, Stock Options.
David Rubin: AbbVie – Consultant. AltruBio – Consultant. Apex – Consultant. Avalo Therapeutics – Consultant. Bausch Health – Consultant. Bristol Myers Squibb – Consultant. Buhlmann Diagnostics Corp – Consultant. Celgene – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health – Board of Directors. Crohn's & Colitis Foundation – Board of Trustees. Douglas Therapeutics – Consultant. Eli Lilly – Consultant. InDex Pharmaceuticals – Consultant. Intouch Group – Consultant. Iterative Health – Consultant. Janssen Pharmaceuticals – Consultant. Odyssey Thera – Consultant. Pfizer – Consultant. Prometheus Biosciences – Consultant. Samsung Neurologica – Consultant. Takeda – Consultant, Grant/Research Support.
Marla Dubinsky, MD1, Miguel D Regueiro, MD2, Christina Ha, MD3, Gil Y. Melmed, MD4, Alessandro Armuzzi, MD, PhD5, Peter M.. Irving, MA, MD6, Samuel Anyanwu, MD7, Elena Dubcenco, MD7, Fernando Aponte, MD7, Isheeta Shah, MD7, Tao Wang, MD7, Smitha Suravaram, MD8, Hannah Palac, MD7, David T.. Rubin, MD, FACG9. P0850 - Effect of Upadacitinib Treatment on Hematological Parameters in Patients With Moderate to Severe Active Crohn’s Disease or Ulcerative Colitis: Pooled Analysis of Phase 3 Induction and Maintenance Studies, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
