Sunday Poster Session
Category: IBD
P0864 - Association Between Fecal Calprotectin Cutoff Concentrations of 150 or 250 μg/g and Ozanimod Efficacy: A Post Hoc Analysis of the Phase 3 True North Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Andres J. Yarur, MD
Associate Professor of Medicine
Inflammatory Bowel Disease Center, Cedars-Sinai Medical Center
Los Angeles, CA
Presenting Author(s)
Andres Yarur, MD1, Marla C. Dubinsky, MD2, Subrata Ghosh, MD3, Razvan Arsenescu, MD, PhD4, Sebastian Zeissig, MD5, Zhaohui Liu, PhD6, Sarah Harris, PhD7, Dimpy Mehra, PharmD6, Anjali Jain, PhD6, Ashwin Ananthakrishnan, MBBS, MPH8
1Cedars-Sinai Medical Center, Los Angeles, CA; 2Icahn School of Medicine at Mount Sinai, New York, NY; 3University College Cork, Cork, Cork, Ireland; 4Atrius Health, Medford, MA; 5Technische Universität Dresden, Dresden, Berlin, Germany; 6Bristol Myers Squibb, Princeton, NJ; 7Bristol Myers Squibb, San Diego, CA; 8Massachusetts General Hospital, Boston, MA
Introduction: Ozanimod (OZA) was effective and well tolerated up to 52 wk in patients (pts) with moderately to severely active ulcerative colitis in the phase 3 True North (TN) study (NCT02435992).1 Fecal calprotectin (FCP) is a surrogate biomarker of gut inflammation that was correlated with OZA efficacy in TN. Data on FCP cutoffs that predict long- and short-term response are important to guide management in clinical practice.
Methods: This analysis assessed the relationships between specific FCP cutoff values at baseline (BL) and Week (W) 10 with OZA efficacy at W10 and W52 in TN. Pts were randomized to placebo (PBO) or OZA (Cohort 1) or to open-label OZA (Cohort 2), and W10 OZA clinical responders were rerandomized to OZA or PBO through W52. BL FCP concentrations were compared in W10 responders vs nonresponders. W10 efficacy outcomes were compared in pts with BL FCP cutoff concentrations of ≤250 μg/g vs >250 μg/g. OZA efficacy at W52 was evaluated in pts whose FCP concentrations reduced below 250 μg/g and 150 μg/g from BL to W10 vs pts whose FCP concentrations remained above these thresholds at W10.
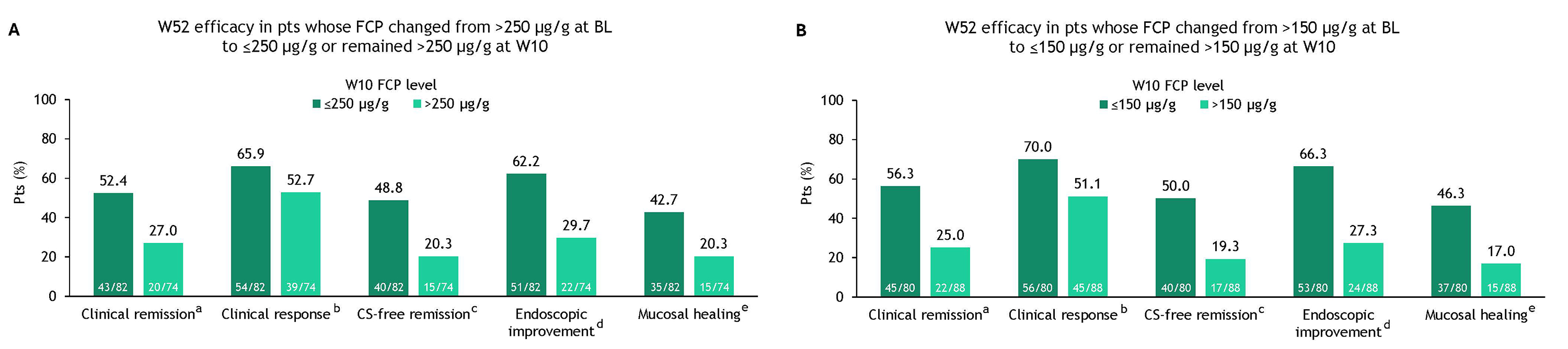
Results: FCP concentrations at TN BL were >250 μg/g in most pts. Median BL FCP concentrations were similar between OZA W10 responders and nonresponders (Cohort 1 OZA: 1097.6 vs 1085.0; Cohort 2 OZA: 1126.6 vs 1320.8, respectively). At W10, treatment differences between OZA and PBO were greater in pts with BL FCP concentrations >250 μg/g vs ≤250 μg/g; PBO-adjusted differences in proportions were 14.0% vs 5.7% (clinical remission), 26.7% vs −0.2% (clinical response), 16.8% vs 10.1% (endoscopic improvement), and 10.1% vs 6.7% (mucosal healing) (Table 1). A decrease in FCP concentrations from >250 μg/g at BL to ≤250 µg/g after 10 wk of OZA treatment was associated with better outcomes after 52 wk of OZA treatment (Figure 1A). A slightly higher proportion of pts achieved OZA-dependent efficacy outcomes at W52 when FCP concentrations were further reduced from >150 μg/g at BL to ≤150 µg/g at W10 (Figure 1B).
Discussion: BL FCP concentrations >250 ug/g were associated with significantly higher efficacy with OZA treatment vs PBO. Reduction in FCP concentrations to ≤250 μg/g and further to ≤150 μg/g after 10 wk of OZA treatment were associated with better achievement of efficacy outcomes at 1 year. Clinically relevant FCP cutoffs could potentially be considered as a long-term prognostic variable for optimal clinical management.
1. Sandborn WJ et al. N Engl J Med. 2021;385:1280-1291.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Andres Yarur, MD1, Marla C. Dubinsky, MD2, Subrata Ghosh, MD3, Razvan Arsenescu, MD, PhD4, Sebastian Zeissig, MD5, Zhaohui Liu, PhD6, Sarah Harris, PhD7, Dimpy Mehra, PharmD6, Anjali Jain, PhD6, Ashwin Ananthakrishnan, MBBS, MPH8. P0864 - Association Between Fecal Calprotectin Cutoff Concentrations of 150 or 250 μg/g and Ozanimod Efficacy: A Post Hoc Analysis of the Phase 3 True North Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Cedars-Sinai Medical Center, Los Angeles, CA; 2Icahn School of Medicine at Mount Sinai, New York, NY; 3University College Cork, Cork, Cork, Ireland; 4Atrius Health, Medford, MA; 5Technische Universität Dresden, Dresden, Berlin, Germany; 6Bristol Myers Squibb, Princeton, NJ; 7Bristol Myers Squibb, San Diego, CA; 8Massachusetts General Hospital, Boston, MA
Introduction: Ozanimod (OZA) was effective and well tolerated up to 52 wk in patients (pts) with moderately to severely active ulcerative colitis in the phase 3 True North (TN) study (NCT02435992).1 Fecal calprotectin (FCP) is a surrogate biomarker of gut inflammation that was correlated with OZA efficacy in TN. Data on FCP cutoffs that predict long- and short-term response are important to guide management in clinical practice.
Methods: This analysis assessed the relationships between specific FCP cutoff values at baseline (BL) and Week (W) 10 with OZA efficacy at W10 and W52 in TN. Pts were randomized to placebo (PBO) or OZA (Cohort 1) or to open-label OZA (Cohort 2), and W10 OZA clinical responders were rerandomized to OZA or PBO through W52. BL FCP concentrations were compared in W10 responders vs nonresponders. W10 efficacy outcomes were compared in pts with BL FCP cutoff concentrations of ≤250 μg/g vs >250 μg/g. OZA efficacy at W52 was evaluated in pts whose FCP concentrations reduced below 250 μg/g and 150 μg/g from BL to W10 vs pts whose FCP concentrations remained above these thresholds at W10.
Results: FCP concentrations at TN BL were >250 μg/g in most pts. Median BL FCP concentrations were similar between OZA W10 responders and nonresponders (Cohort 1 OZA: 1097.6 vs 1085.0; Cohort 2 OZA: 1126.6 vs 1320.8, respectively). At W10, treatment differences between OZA and PBO were greater in pts with BL FCP concentrations >250 μg/g vs ≤250 μg/g; PBO-adjusted differences in proportions were 14.0% vs 5.7% (clinical remission), 26.7% vs −0.2% (clinical response), 16.8% vs 10.1% (endoscopic improvement), and 10.1% vs 6.7% (mucosal healing) (Table 1). A decrease in FCP concentrations from >250 μg/g at BL to ≤250 µg/g after 10 wk of OZA treatment was associated with better outcomes after 52 wk of OZA treatment (Figure 1A). A slightly higher proportion of pts achieved OZA-dependent efficacy outcomes at W52 when FCP concentrations were further reduced from >150 μg/g at BL to ≤150 µg/g at W10 (Figure 1B).
Discussion: BL FCP concentrations >250 ug/g were associated with significantly higher efficacy with OZA treatment vs PBO. Reduction in FCP concentrations to ≤250 μg/g and further to ≤150 μg/g after 10 wk of OZA treatment were associated with better achievement of efficacy outcomes at 1 year. Clinically relevant FCP cutoffs could potentially be considered as a long-term prognostic variable for optimal clinical management.
1. Sandborn WJ et al. N Engl J Med. 2021;385:1280-1291.

Figure: Figure 1. TN W52 OZA efficacy by changes in FCP concentration from BL to W10 using a cutoff of (A) 250 μg/g and (B) 150 μg/g in pts receiving continuous OZA in TN. aClinical remission: RBS = 0 point and SFS ≤1 point (and a decrease of ≥1 point from the BL SFS) and MES ≤1 point. bClinical response: a reduction from BL in the 9-point Mayo score of ≥2 points and ≥35%, and a reduction from BL in the RBS of ≥1 point or an absolute RBS of ≤1 point. cCS-free remission: clinical remission at 52 wk while off CS for ≥12 wk. dEndoscopic improvement: MES ≤1 point without friability. eMucosal healing: MES ≤1 point and Geboes score <2.0. BL, baseline; CS, corticosteroid; FCP, fecal calprotectin; MES, Mayo endoscopy subscore; OZA, ozanimod; pt, patient; RBS, rectal bleeding subscore; SFS, stool frequency subscore; TN, True North; W, Week.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Andres Yarur: AbbVie – Consultant, served on clinical trial steering committee. Abivax – Advisory Committee/Board Member, Consultant. Arena – Consultant, served on clinical trial steering committee. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Consultant, served on clinical trial steering committee. Celltrion – Consultant, served on clinical trial steering committee. Johnson and Johnson – Advisory Committee/Board Member, Consultant. Pfizer – Consultant, served on clinical trial steering committee. Takeda – Consultant, served on clinical trial steering committee.
Marla Dubinsky: AbbVie – Consultant. Abivax – Consultant. Arena – Consultant. AstraZeneca – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Genentech – Consultant. Gilead – Consultant. Janssen – Consultant. Pfizer Inc – Consultant. Prometheus Labs – Consultant. Takeda – Consultant.
Subrata Ghosh: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Bristol Myers Squibb – Consultant. Celgene – Speakers Bureau. Eli Lilly – Speakers Bureau. Ferring – Speakers Bureau. Galapagos – Speakers Bureau. Gilead – Speakers Bureau. GlaxoSmithKline – Grant/Research Support. Janssen – Consultant, Speakers Bureau. MSD – Speakers Bureau. Novo Nordisk – Consultant. Pfizer – Consultant, Speakers Bureau. Roche – Consultant. Takeda – Consultant, Speakers Bureau.
Razvan Arsenescu indicated no relevant financial relationships.
Sebastian Zeissig: AbbVie Deutschland GmbH – Consultant, Speakers Bureau. Amgen GmbH – Consultant, Speakers Bureau. Biogen GmbH – Consultant, Speakers Bureau. Bristol Myers Squibb GmbH – Consultant, Speakers Bureau. CED Service GmbH – Speakers Bureau. Celgene GmbH – Consultant. Celltrion – Consultant. Falk Foundation – Speakers Bureau. Ferring Arzneimittel GmbH – Consultant, Speakers Bureau. FORGA Solutions GmbH – Speakers Bureau. Galapagos Biopharma Germany GmbH – Consultant. GWT-TUD GmbH – Speakers Bureau. Hogg Robinson GmbH – Consultant. Janssen Cilag GmbH – Consultant, Speakers Bureau. Janssen Pharmaceutica NV – Consultant, Speakers Bureau. Merck Sharp & Dohme – Speakers Bureau. MICE Service GmbH – Consultant. Mylan – Consultant. Pfizer Pharma GmbH – Consultant, Speakers Bureau. Pharma-Zentrale GmbH – Speakers Bureau. Roche – Speakers Bureau. Takeda Pharma GmbH – Consultant, Speakers Bureau. WebMD Global – Speakers Bureau.
Zhaohui Liu: Bristol Myers Squibb – Employee.
Sarah Harris: Bristol Myers Squibb – Employee.
Dimpy Mehra: Bristol Myers Squibb – Employee.
Anjali Jain: Bristol Myers Squibb – Employee.
Ashwin Ananthakrishnan: Geneoscopy – Advisor or Review Panel Member.
Andres Yarur, MD1, Marla C. Dubinsky, MD2, Subrata Ghosh, MD3, Razvan Arsenescu, MD, PhD4, Sebastian Zeissig, MD5, Zhaohui Liu, PhD6, Sarah Harris, PhD7, Dimpy Mehra, PharmD6, Anjali Jain, PhD6, Ashwin Ananthakrishnan, MBBS, MPH8. P0864 - Association Between Fecal Calprotectin Cutoff Concentrations of 150 or 250 μg/g and Ozanimod Efficacy: A Post Hoc Analysis of the Phase 3 True North Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
