Sunday Poster Session
Category: IBD
P0896 - A Randomized, Double-Blind, Placebo-Controlled Trial of Vedolizumab With and Without Upadacitinib in Adults With Crohn’s Disease: Design and Rationale for the VICTRIVA Study
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio
- PO
Pooja Oberai, MD
Takeda
Cambridge, MA
Presenting Author(s)
Silvio Danese, MD, PhD1, Bruce E.. Sands, MD, FACG2, Brian G.. Feagan, MD3, Vipul Jairath, MBChB3, Remo Panaccione, MD4, Laurent Peyrin-Biroulet, MD, PhD5, Peter M.. Irving, MA, MD6, Stefan Schreiber, MD7, Iris Dotan, MD8, Marc Ferrante, MD, PhD9, Geert R. D'Haens, MD, PhD10, Stephen Jones, MBBS, BSc11, Marcelo Freire, PhD12, Dirk Lindner, MSc13, Shashi Adsul, MD, MBA12, Pooja Oberai, MD12, Jean-Frédéric Colombel, MD2
1Humanitas Clinical and Research Center - IRCCS, Rozzano and Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 2Icahn School of Medicine at Mount Sinai, New York, NY; 3Western University, London, ON, Canada; 4University of Calgary, Calgary, AB, Canada; 5INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 6Guy’s and St. Thomas’ NHS Foundation Trust, London, England, United Kingdom; 7University Hospital, Kiel, Schleswig-Holstein, Germany; 8Rabin Medical Center, Tel Aviv University, Tel Aviv, Tel Aviv, Israel; 9University Hospitals, Leuven, Vlaams-Brabant, Belgium; 10Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands; 11Takeda, UK, England, United Kingdom; 12Takeda, Cambridge, MA; 13Takeda, Zurich, Zurich, Switzerland
Introduction: Remission rates in patients with Crohn’s disease (CD) suggest a therapeutic ceiling when treated with a single advanced targeted treatment (ATT) or ATT plus immunomodulator, representing an unmet need for alternative strategies. Dual targeted therapy (DTT)/advanced combination therapy has potential to provide an additive or synergistic benefit by targeting >1 pathway. Vedolizumab (VDZ) and upadacitinib (UPA) are treatments for moderate to severe CD with different mechanisms of action, and in silico models suggest their combination could modulate a high number of pathways relevant to the pathophysiology of CD. The VICTRIVA trial aims to compare the efficacy and safety of induction with VDZ + UPA vs VDZ alone.
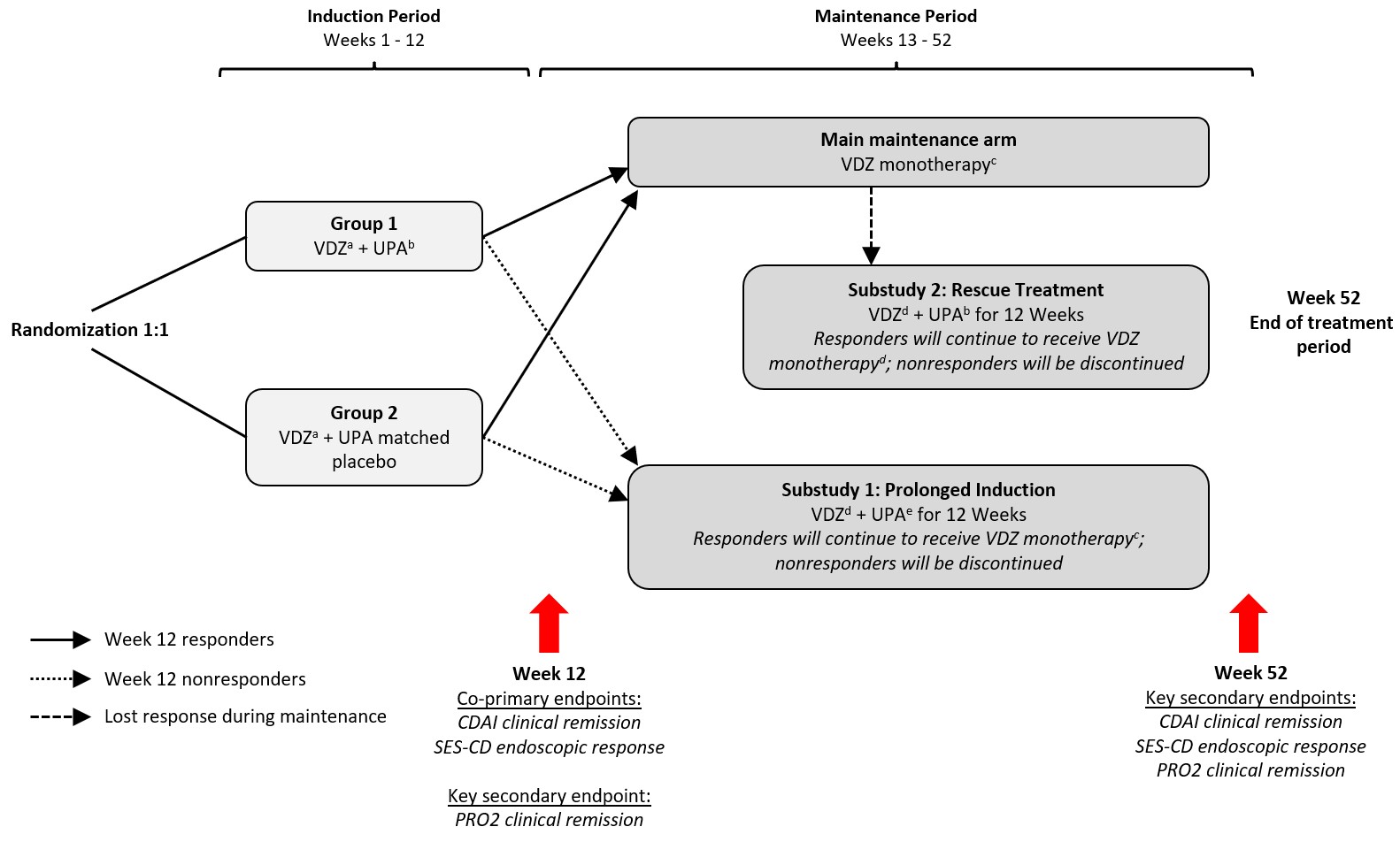
Methods: The primary objective of VICTRIVA (NCT06227910), a randomized, double-blind, controlled, phase 3b trial in biologic experienced and biologic naïve adult patients with CD, is to assess whether induction with VDZ + UPA improves rates of clinical remission and endoscopic response at week(W) 12 vs VDZ alone. Patients will be randomized 1:1 to 12 week induction with VDZ + UPA (Group 1) or VDZ + placebo (Group 2) (Figure). W12 responders will enter the maintenance arm from W13-52 (VDZ monotherapy every 8 weeks [Q8W] to W52; possible Q4W escalation if needed). W12 nonresponders will enter prolonged induction Substudy 1 (VDZ + UPA to W24, followed by VDZ monotherapy). Patients who lose response during maintenance will be escalated to Q4W, or enter rescue treatment Substudy 2 (DTT for 12 weeks, then VDZ Q4W). Assessments include Crohn’s Disease Activity Index (CDAI) and patient reported outcomes at W2,6,12 during induction and in the maintenance and substudies through W52, Simple Endoscopic Score for Crohn’s Disease (SES-CD) at screening, W12 and 52, and safety at each visit.
Results: 396 patients (198 in Groups 1 and 2) will be enrolled globally. Co-primary endpoints will be CDAI clinical remission (score < 150) and SES-CD endoscopic response ( >50% score reduction) at W12. Key secondary endpoints include PRO2 clinical remission at W12, and CDAI clinical remission, SES-CD endoscopic response and PRO2 clinical remission at W52. Safety endpoints will include adverse events and adverse events of special interest.
Discussion: The VICTRIVA trial is designed to evaluate the efficacy and safety of VDZ in combination with UPA relative to VDZ monotherapy in an attempt to break the current therapeutic ceiling for inducing remission in CD and improve long-term outcomes.
Disclosures:
Silvio Danese, MD, PhD1, Bruce E.. Sands, MD, FACG2, Brian G.. Feagan, MD3, Vipul Jairath, MBChB3, Remo Panaccione, MD4, Laurent Peyrin-Biroulet, MD, PhD5, Peter M.. Irving, MA, MD6, Stefan Schreiber, MD7, Iris Dotan, MD8, Marc Ferrante, MD, PhD9, Geert R. D'Haens, MD, PhD10, Stephen Jones, MBBS, BSc11, Marcelo Freire, PhD12, Dirk Lindner, MSc13, Shashi Adsul, MD, MBA12, Pooja Oberai, MD12, Jean-Frédéric Colombel, MD2. P0896 - A Randomized, Double-Blind, Placebo-Controlled Trial of Vedolizumab With and Without Upadacitinib in Adults With Crohn’s Disease: Design and Rationale for the VICTRIVA Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Humanitas Clinical and Research Center - IRCCS, Rozzano and Humanitas University, Pieve Emanuele, Milan, Lombardia, Italy; 2Icahn School of Medicine at Mount Sinai, New York, NY; 3Western University, London, ON, Canada; 4University of Calgary, Calgary, AB, Canada; 5INFINY Institute, FHU-CURE, INSERM NGERE, Nancy University Hospital, Vandœuvre-lès-Nancy, Lorraine, France; McGill University Health Centre, Montreal, QC, Canada, Nancy, Lorraine, France; 6Guy’s and St. Thomas’ NHS Foundation Trust, London, England, United Kingdom; 7University Hospital, Kiel, Schleswig-Holstein, Germany; 8Rabin Medical Center, Tel Aviv University, Tel Aviv, Tel Aviv, Israel; 9University Hospitals, Leuven, Vlaams-Brabant, Belgium; 10Amsterdam University Medical Center, Amsterdam, Limburg, Netherlands; 11Takeda, UK, England, United Kingdom; 12Takeda, Cambridge, MA; 13Takeda, Zurich, Zurich, Switzerland
Introduction: Remission rates in patients with Crohn’s disease (CD) suggest a therapeutic ceiling when treated with a single advanced targeted treatment (ATT) or ATT plus immunomodulator, representing an unmet need for alternative strategies. Dual targeted therapy (DTT)/advanced combination therapy has potential to provide an additive or synergistic benefit by targeting >1 pathway. Vedolizumab (VDZ) and upadacitinib (UPA) are treatments for moderate to severe CD with different mechanisms of action, and in silico models suggest their combination could modulate a high number of pathways relevant to the pathophysiology of CD. The VICTRIVA trial aims to compare the efficacy and safety of induction with VDZ + UPA vs VDZ alone.
Methods: The primary objective of VICTRIVA (NCT06227910), a randomized, double-blind, controlled, phase 3b trial in biologic experienced and biologic naïve adult patients with CD, is to assess whether induction with VDZ + UPA improves rates of clinical remission and endoscopic response at week(W) 12 vs VDZ alone. Patients will be randomized 1:1 to 12 week induction with VDZ + UPA (Group 1) or VDZ + placebo (Group 2) (Figure). W12 responders will enter the maintenance arm from W13-52 (VDZ monotherapy every 8 weeks [Q8W] to W52; possible Q4W escalation if needed). W12 nonresponders will enter prolonged induction Substudy 1 (VDZ + UPA to W24, followed by VDZ monotherapy). Patients who lose response during maintenance will be escalated to Q4W, or enter rescue treatment Substudy 2 (DTT for 12 weeks, then VDZ Q4W). Assessments include Crohn’s Disease Activity Index (CDAI) and patient reported outcomes at W2,6,12 during induction and in the maintenance and substudies through W52, Simple Endoscopic Score for Crohn’s Disease (SES-CD) at screening, W12 and 52, and safety at each visit.
Results: 396 patients (198 in Groups 1 and 2) will be enrolled globally. Co-primary endpoints will be CDAI clinical remission (score < 150) and SES-CD endoscopic response ( >50% score reduction) at W12. Key secondary endpoints include PRO2 clinical remission at W12, and CDAI clinical remission, SES-CD endoscopic response and PRO2 clinical remission at W52. Safety endpoints will include adverse events and adverse events of special interest.
Discussion: The VICTRIVA trial is designed to evaluate the efficacy and safety of VDZ in combination with UPA relative to VDZ monotherapy in an attempt to break the current therapeutic ceiling for inducing remission in CD and improve long-term outcomes.

Figure: aVDZ IV 300 mg at Weeks 0, 2, 6, and 10. bUPA oral 45 mg once daily. cVDZ IV 300 mg Q8W, possible escalation to Q4W. dVDZ IV 300 mg Q4W. eUPA oral 30 mg once daily for patients initially assigned to Group 1, 45 mg once daily for patients initially assigned to Group 2.
CDAI, Crohn’s Disease Activity Index; PRO2, Patient Reported Outcome; SES-CD, Simple Endoscopic Score for Crohn’s Disease; UPA, upadacitinib; VDZ, vedolizumab.
CDAI, Crohn’s Disease Activity Index; PRO2, Patient Reported Outcome; SES-CD, Simple Endoscopic Score for Crohn’s Disease; UPA, upadacitinib; VDZ, vedolizumab.
Disclosures:
Silvio Danese: AbbVie – Consultant, Speakers Bureau. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Speakers Bureau. Applied Molecular Transport – Consultant. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Celltrion Healthcare – Consultant. Dr Falk Pharma – Consultant. Eli Lilly and Company – Consultant. Enthera – Consultant. Ferring – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. Hospira – Consultant. Inotrem – Consultant. Janssen – Consultant, Speakers Bureau. Johnson & Johnson – Consultant. Morphic – Consultant. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Speakers Bureau. Pfizer Inc – Consultant, Speakers Bureau. Roche – Consultant. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Speakers Bureau. Teladoc Health – Consultant. TiGenix – Consultant. UCB Inc. – Consultant. Vial – Consultant. Vifor – Consultant.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Agomab – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Other support, Speakers Bureau. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Enthera – Consultant. Envied Biosciences – Consultant. Equilium – Consultant. Evommune – Consultant. Ferring – Consultant. Fiat – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. Glaxo SmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen – Consultant, Grant/Research Support, Other support, Speakers Bureau. Kaleido – Consultant. Kallyope – Consultant. Lilly – Consultant, other support, Speakers Bureau. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, Other support, Speakers Bureau. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, Other support, Speakers Bureau. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biopharma – Consultant, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Brian Feagan: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. AbolerIS – Consultant. AgomAB Therapeutics – Consultant. Allianthera – Consultant. Amgen – Advisory Committee/Board Member, Consultant. AnaptysBio – Advisory Committee/Board Member, Consultant. Applied Molecular Transport Inc – Advisory Committee/Board Member, Consultant. Arena Pharma – Consultant. Atomwise – Consultant. Avoro Capital Advisors – Consultant. Axio Research – Advisory Committee/Board Member. BioJamp – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Boxer – Consultant. Celgene/Bristol Myers Squibb – Advisory Committee/Board Member, Consultant. Celsius Therapeutics – Consultant. Connect BioPharma – Consultant, stock or other ownership interest. Cytoki – Consultant. Disc Medicine – Consultant. Duality – Consultant. EcoR1 Capital – Advisory Committee/Board Member, Consultant. Equillium – Consultant. Ermium – Consultant. First Wave – Consultant. First Word Group – Consultant. Galapagos – Consultant. Galen Atlantica – Consultant. Genentech/Roche – Advisory Committee/Board Member, Consultant. Gilead – Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Gossamer Pharma – Consultant, Stock Options. Hinge Bio – Consultant. Hot Spot Therapeutics – Consultant. Imhotex – Consultant. Immunic Therapeutics – Consultant. InDex Pharmaceuticals – Advisory Committee/Board Member, Consultant. JAKAcademy – Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Japan Tobacco Inc. – Consultant. Kaleido Biosciences – Consultant. L.E.K. Consulting – Consultant. Landos Biopharma – Consultant. Leadiant – Consultant. Lenczner Slaght – Consultant, payment for expert testimony. LifeSci Capital – Consultant. Lilly – Advisory Committee/Board Member, Consultant. Lument AB – Consultant. Millennium – Consultant. MiroBio – Advisory Committee/Board Member, Consultant. Morgan Lewis – Consultant, payment for expert testimony. Morphic Therapeutics – Advisory Committee/Board Member, Consultant. Mylan – Consultant. OM Pharma – Consultant. Origo BioPharma – Advisory Committee/Board Member, Consultant. Orphagen – Consultant. Pandion Therapeutics – Consultant. Pendopharm – Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Grant/Research Support. Play to Know AG – Consultant. Progenity – Advisory Committee/Board Member, Consultant. Prometheus – Advisory Committee/Board Member, Consultant. Protagonist – Consultant. PTM Therapeutics – Consultant. Q32 Bio – Consultant. Rebiotix – Consultant. REDX – Advisory Committee/Board Member, Consultant. Roche – Consultant. Sandoz – Consultant. Sanofi – Advisory Committee/Board Member, Consultant. Seres Therapeutics – Consultant. Silverback Therapeutics – Consultant. Surrozen Inc. – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Teva – Advisory Committee/Board Member, Consultant. Thelium – Consultant. Tigenix – Consultant. Tillotts Pharma – Advisory Committee/Board Member, Consultant. Ventyx Biosciences – Consultant. VHSquared Ltd – Consultant. Viatris – Consultant. Ysios – Consultant. Ysopia – Consultant. Zealand Pharma – Consultant.
Vipul Jairath: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Alimentiv – Consultant, Employee, Grant/Research Support, Speakers Bureau. Arena Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Pharma – Consultant, Grant/Research Support, Speakers Bureau. Asieris Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Consultant, Grant/Research Support, Speakers Bureau. Avoro Capital – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly and Company – Consultant, Grant/Research Support, Speakers Bureau. Endpoint Health – Advisory Committee/Board Member, Consultant. Enthera – Advisory Committee/Board Member, Consultant. Ferring Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Flagship Pioneering – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Grant/Research Support, Speakers Bureau. Galapagos NV – Consultant, Grant/Research Support, Speakers Bureau. Genentech – Consultant, Grant/Research Support, Speakers Bureau. Gilde Healthcare – Advisory Committee/Board Member, Consultant. Gilead Sciences – Consultant, Grant/Research Support, Speakers Bureau. GlaxoSmithKline – Consultant, Grant/Research Support, Speakers Bureau. Innomar – Advisory Committee/Board Member, Consultant. JAMP – Advisory Committee/Board Member, Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. London Health Sciences Centre – Employee. Merck – Consultant, Grant/Research Support, Speakers Bureau. Metacrine – Consultant, Grant/Research Support, Speakers Bureau. Mylan – Consultant, Grant/Research Support, Speakers Bureau. Pandion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Pendopharm – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Therapeutics and Diagnostics – Consultant, Grant/Research Support, Speakers Bureau. Protagonist Therapeutics – Consultant, Grant/Research Support, Speakers Bureau. Reistone Biopharma – Consultant, Grant/Research Support, Speakers Bureau. Roche – Consultant, Grant/Research Support, Speakers Bureau. Roivant – Advisory Committee/Board Member, Consultant. Sandoz – Consultant, Grant/Research Support, Speakers Bureau. SCOPE – Advisory Committee/Board Member, Consultant. Second Genome – Consultant, Grant/Research Support, Speakers Bureau. Shire – Speakers Bureau. Sorriso Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Synedgen – Advisory Committee/Board Member, Consultant. Takeda – Consultant, Grant/Research Support, Speakers Bureau. TD Securities – Advisory Committee/Board Member, Consultant. Teva – Consultant, Grant/Research Support, Speakers Bureau. Topivert – Consultant, Grant/Research Support, Speakers Bureau. Ventyx Biosciences – Consultant, Grant/Research Support, Speakers Bureau. Vividion Therapeutics – Consultant, Grant/Research Support, Speakers Bureau.
Remo Panaccione: Élan – Consultant. Abbivax – Consultant. Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaking Fees. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker Fees. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker Fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker Fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker Fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Ferring – Advisory Committee/Board Member, Consultant, Speaker Fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker Fees. Galapagos – Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant, Speaker Fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Bio – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speaker Fees. Lilly – Advisory Committee/Board Member, Consultant, Speaker Fees. Merck – Advisory Committee/Board Member, Consultant, Speaker Fees. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker Fees. Pandion Pharma – Advisory Committee/Board Member, Consultant. Pendopharm – Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Speaker Fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker Fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker Fees. Satisfai Health – Consultant. Shire – Advisory Committee/Board Member, Consultant, Speaker Fees. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speaker Fees. Theravance – Consultant. Trellus – Consultant. UCB – Consultant. Ventyx – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Laurent Peyrin-Biroulet: AbbVie – Grant/Research Support, Personal fees. Allergan – Personal Fees. Alma Bio Therapeutics – Personal Fees. Amgen – Personal Fees. Applied Molecular Transport – Personal Fees. Arena – Personal Fees. Biogen – Personal Fees. Boehringer Ingelheim – Personal Fees. Bristol Myers Squibb – Personal Fees. Celgene – Personal Fees. Celltrion – Personal Fees. CTMA – Stock Options. Enterome – Personal Fees. Enthera – Personal Fees. Ferring – Personal Fees. Fresenius Kabi – Personal Fees. Genentech – Personal Fees. Gilead – Personal Fees. Hikma – Personal Fees. InDex Pharmaceuticals – Personal Fees. Janssen – Personal Fees. Lilly – Personal Fees. MSD – Grant/Research Support, Personal Fees. Mylan – Personal Fees. Nestlé – Personal Fees. Norgine – Personal Fees. Oppilan Pharma – Personal Fees. OSE Immunotherapeutics – Personal Fees. Pfizer Inc – Personal Fees. Pharmacosmos – Fees. Samsung Bioepis – Personal Fees. Sandoz – Personal Fees. Sterna – Personal Fees. Sublimity Therapeutics – Personal Fees. Takeda – Grant/Research Support, Personal Fees. Tillotts – Personal Fees. Vifor – Personal Fees.
Peter Irving: AbbVie – Advisory Committee/Board Member, Speakers Bureau. Arena – Advisory Committee/Board Member. Boehringer Ingelheim – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celgene – Advisory Committee/Board Member, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Dr. Falk Pharma – Speakers Bureau. Eli Lilly – Advisory Committee/Board Member. Ferring – Speakers Bureau. Galapagos – Speakers Bureau. Genentech – Advisory Committee/Board Member. Gilead – Advisory Committee/Board Member, Speakers Bureau. Hospira – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Speakers Bureau. MSD – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Pharmacosmos – Advisory Committee/Board Member. Prometheus Biosciences – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member. Samsung Bioepis – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member, Speakers Bureau. Sapphire Medical – Speakers Bureau. Shire – Speakers Bureau. Takeda – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Tillotts – Speakers Bureau. TopiVert – Advisory Committee/Board Member. VH2 – Advisory Committee/Board Member. Vifor – Advisory Committee/Board Member. Warner Chilcott – Advisory Committee/Board Member, Speakers Bureau.
Stefan Schreiber: AbbVie – Consultant, Personal fees, Speakers Bureau. Amgen – Personal fees. Arena Pharmaceuticals – Consultant, Personal fees, Speakers Bureau. Biogen – Consultant, Personal fees, Speakers Bureau. Bristol Myers Squibb – Consultant, Personal fees, Speakers Bureau. Celgene – Consultant, Personal fees, Speakers Bureau. Celltrion – Consultant, Personal fees, Speakers Bureau. Eli Lilly and Company – Personal fees. Falk – Consultant, Personal fees, Speakers Bureau. Ferring Pharmaceuticals – Personal fees. Fresenius – Consultant, Personal fees, Speakers Bureau. Galapagos – Personal fees. Gilead – Consultant, Personal fees. Hikma Pharmaceuticals – Advisory Committee/Board Member, Consultant. I-MAB – Consultant, Personal fees. Janssen – Consultant, Personal fees, Speakers Bureau. Morphic – Personal fees. MSD – Consultant, Personal fees, Speakers Bureau. Mylan – Consultant, Personal fees. Novartis – Personal fees. Pfizer Inc – Consultant, Personal fees, Speakers Bureau. Protagonist – Consultant, Personal fees. Provention Bio – Consultant, Personal fees. Roche – Personal fees. Sandoz/Hexal – Personal fees. Shire – Personal fees. Takeda – Consultant, Personal fees, Speakers Bureau. Theravance Biopharma – Consultant, Personal fees. Ventyx – Consultant, Personal fees.
Iris Dotan: Abbvie – Advisory Committee/Board Member, Consultant, Speakers Bureau. Altman Research – Advisory Committee/Board Member, Consultant, Speakers Bureau. Arena – Advisory Committee/Board Member, Consultant, Speakers Bureau. Athos – Advisory Committee/Board Member, Consultant, Speakers Bureau. Cambridge Healthcare – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celgene/BMS – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Falk Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Galapagos – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gutreat – Shareholder. Harp Dx – Advisory Committee/Board Member, Consultant, Shareholder, Speakers Bureau. Integra Holdings – Advisory Committee/Board Member, Consultant, Speakers Bureau. Iterative Scopes – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Kenes – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Neopharm – Advisory Committee/Board Member, Consultant, Speakers Bureau. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Prometheus – Advisory Committee/Board Member, Consultant, Speakers Bureau. Roche/Genentech – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sangamo – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sublimity – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Marc Ferrante: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Agomab – Consultant. Amgen – Grant/Research Support, Speakers Bureau. Biogen – Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Celgene – Consultant. Celltrion – Consultant. Dr Falk Pharma – Speakers Bureau. EG Pharmaceuticals – Grant/Research Support. Eli Lilly and Company – Consultant, Grant/Research Support. Ferring – Speakers Bureau. Janssen – Grant/Research Support. Janssen-Cilag – Consultant, Speakers Bureau. Lamepro – Speakers Bureau. Medtronic – Consultant. MRM Health – Consultant. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Regeneron – Consultant. Samsung Bioepis – Consultant. Sandoz – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. ThermoFisher – Consultant. Truvion Healthcare – Speakers Bureau. Viatris – Grant/Research Support, Speakers Bureau.
Geert D'Haens: AbbVie – Advisor or Review Panel Member, Speakers Bureau. Agomab Therapeutics – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. Allergan – Advisor or Review Panel Member. Alphabiomics – Advisor or Review Panel Member. AstraZeneca – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Speakers Bureau. Ferring – Advisor or Review Panel Member. Galapagos – Advisor or Review Panel Member, Speakers Bureau. GlaxoSmithKline – Advisor or Review Panel Member. Immunic – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Seres – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau. Tillotts – Advisor or Review Panel Member, Speakers Bureau. Ventyx – Advisor or Review Panel Member.
Stephen Jones: Takeda – Consultant.
Marcelo Freire: Takeda – Employee, Stock Options.
Dirk Lindner: Takeda – Employee, Stock Options.
Shashi Adsul: Takeda – Employee, Stock Options.
Pooja Oberai: Takeda – Employee, Stock Options.
Jean-Frédéric Colombel: AbbVie – Consultant, Grant/Research Support. Allergan – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squib – Consultant, Grant/Research Support. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enterome – Consultant. Ferring – Consultant. Galmed Research – Consultant. Genentech – Consultant. Genfit – Stock-publicly held company(excluding mutual/index funds). Glaxo Smith Kline – Consultant. Immunic – Consultant. Intestinal Biotech Development – Stock-publicly held company(excluding mutual/index funds). Iterative Scopes – Consultant. Janssen – Consultant, Grant/Research Support. Johnson & Johnson – Consultant, Grant/Research Support. Kaleido Biosciences – Consultant. Landos – Consultant. MedImmune – Consultant. Merck – Consultant. Microba Life Science – Consultant. Novartis – Consultant. Otsuka Pharmaceutical – Consultant. Pfizer – Consultant. PPM Services – Consultant. Protagonist – Consultant. Sanofi – Consultant. Second Genome – Consultant. Seres – Consultant. Shire – Consultant. Takeda – Consultant, Grant/Research Support. Theradiag – Consultant. TiGenix – Consultant. Vifor – Consultant.
Silvio Danese, MD, PhD1, Bruce E.. Sands, MD, FACG2, Brian G.. Feagan, MD3, Vipul Jairath, MBChB3, Remo Panaccione, MD4, Laurent Peyrin-Biroulet, MD, PhD5, Peter M.. Irving, MA, MD6, Stefan Schreiber, MD7, Iris Dotan, MD8, Marc Ferrante, MD, PhD9, Geert R. D'Haens, MD, PhD10, Stephen Jones, MBBS, BSc11, Marcelo Freire, PhD12, Dirk Lindner, MSc13, Shashi Adsul, MD, MBA12, Pooja Oberai, MD12, Jean-Frédéric Colombel, MD2. P0896 - A Randomized, Double-Blind, Placebo-Controlled Trial of Vedolizumab With and Without Upadacitinib in Adults With Crohn’s Disease: Design and Rationale for the VICTRIVA Study, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
