Sunday Poster Session
Category: Liver
P1187 - Exploring the Limits: Minimizing Immunosuppression and Its Adverse Effects
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E

Has Audio

Abdulmalik Saleem, MD
Henry Ford Health
Flushing, MI
Presenting Author(s)
Abdulmalik Saleem, MD1, Saleh Al-Juburi, BS2, Ahmad Alomari, MD3, Ammad Javaid. Chaudhary, MD1, Mohammed Abusuliman, MD1, Muhammad Saad Faisal, MD1, Haya Omeish, MD1, Momin Samad, MD4, Mohammed Nayeem, MD5, Sheema Rehman, DO3, Abu Fahad Abbasi, MD6, Syed-Mohammed Jafri, MD1
1Henry Ford Health, Detroit, MI; 2Wayne State University School of Medicine, Detroit, MI; 3Henry Ford Hospital, Detroit, MI; 4Henry Ford Hospital, Rochester Hills, MI; 5Parkview Medical Center, Pueblo, CO; 6Mercy Health, Rockford, IL
Introduction: Post liver transplantation survival has substantially improved over the last few decades. Post-transplant management remains particularly challenging due to the intricate balance between immunosuppression and its adverse effects. Excess immunosuppression can be associated with metabolic disease, infection, and malignancy. Our study aims to delineate this balance to guide clinicians in their ability to safely minimize immunosuppression without predisposing to rejection.
Methods: Our study included patients who have had a 10-year liver transplant course starting in 2013 and were on tacrolimus immunosuppression. Patients who expired between 2018 – 2023 and those without adequate clinical data were excluded. Mean 5-year tacrolimus troughs 5 years after transplant (2018 – 2023) were calculated. A mean trough level of 4 was used as a cutoff to subcategorize our patients into those with a 5 year mean trough of ≤4 or >4. We compared the incidence of rejection, infection, malignancies, hyperkalemia, and nephrotoxicity between these two groups during this time period.
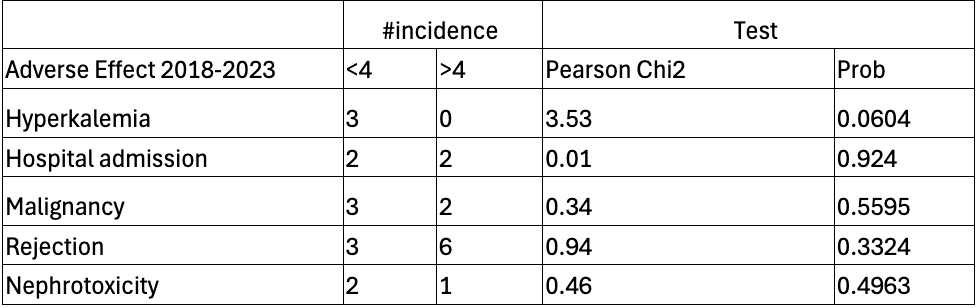
Results: 79 patients underwent liver transplants at our center in 2013. 33 expired or had inadequate data and were excluded. 44 patients met inclusion criteria, 17 Females (38%), 27 Males (61%). The mean age for our patients was 54.6 with a standard deviation of 8.9. Etiologies of cirrhosis were Hepatitis C (45.45%), Alcohol (18.18%), Nonalcoholic Steatohepatitis (18.8%), Cryptogenic (9.09%), Primary Biliary Cirrhosis (4.55%), Primary Sclerosing Cholangitis (2.27%), and Cystic Fibrosis (2.27%). 23 (52%) patients had a mean 5-year tacrolimus trough greater than 4 and 21 (48%) less than 4. 3 patients experienced rejection in the >4 group and 1 in the < 4 group. 2 patients in each group were hospitalized for infection. 2 patients developed a malignancy in the > 4 group and 3 in the < 4 group. 2 patients developed nephrotoxicity in the < 4 group and 1 patient in the >4 group. No significant relationship between the mean 5-year trough and incidence of rejection, hyperkalemia, infection, cancer, and nephrotoxicity was found in our analysis (Table 1).
Discussion: Our findings underscore clinicians’ abilities to down titrate tacrolimus levels without an associated increased risk of rejection. Higher tacrolimus levels were also not associated with an increased incidence of cancer, infections, nephrotoxicity, or hyperkalemia. This discussion may benefit from larger sample sizes for increased generalizability.
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Abdulmalik Saleem, MD1, Saleh Al-Juburi, BS2, Ahmad Alomari, MD3, Ammad Javaid. Chaudhary, MD1, Mohammed Abusuliman, MD1, Muhammad Saad Faisal, MD1, Haya Omeish, MD1, Momin Samad, MD4, Mohammed Nayeem, MD5, Sheema Rehman, DO3, Abu Fahad Abbasi, MD6, Syed-Mohammed Jafri, MD1. P1187 - Exploring the Limits: Minimizing Immunosuppression and Its Adverse Effects, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Henry Ford Health, Detroit, MI; 2Wayne State University School of Medicine, Detroit, MI; 3Henry Ford Hospital, Detroit, MI; 4Henry Ford Hospital, Rochester Hills, MI; 5Parkview Medical Center, Pueblo, CO; 6Mercy Health, Rockford, IL
Introduction: Post liver transplantation survival has substantially improved over the last few decades. Post-transplant management remains particularly challenging due to the intricate balance between immunosuppression and its adverse effects. Excess immunosuppression can be associated with metabolic disease, infection, and malignancy. Our study aims to delineate this balance to guide clinicians in their ability to safely minimize immunosuppression without predisposing to rejection.
Methods: Our study included patients who have had a 10-year liver transplant course starting in 2013 and were on tacrolimus immunosuppression. Patients who expired between 2018 – 2023 and those without adequate clinical data were excluded. Mean 5-year tacrolimus troughs 5 years after transplant (2018 – 2023) were calculated. A mean trough level of 4 was used as a cutoff to subcategorize our patients into those with a 5 year mean trough of ≤4 or >4. We compared the incidence of rejection, infection, malignancies, hyperkalemia, and nephrotoxicity between these two groups during this time period.
Results: 79 patients underwent liver transplants at our center in 2013. 33 expired or had inadequate data and were excluded. 44 patients met inclusion criteria, 17 Females (38%), 27 Males (61%). The mean age for our patients was 54.6 with a standard deviation of 8.9. Etiologies of cirrhosis were Hepatitis C (45.45%), Alcohol (18.18%), Nonalcoholic Steatohepatitis (18.8%), Cryptogenic (9.09%), Primary Biliary Cirrhosis (4.55%), Primary Sclerosing Cholangitis (2.27%), and Cystic Fibrosis (2.27%). 23 (52%) patients had a mean 5-year tacrolimus trough greater than 4 and 21 (48%) less than 4. 3 patients experienced rejection in the >4 group and 1 in the < 4 group. 2 patients in each group were hospitalized for infection. 2 patients developed a malignancy in the > 4 group and 3 in the < 4 group. 2 patients developed nephrotoxicity in the < 4 group and 1 patient in the >4 group. No significant relationship between the mean 5-year trough and incidence of rejection, hyperkalemia, infection, cancer, and nephrotoxicity was found in our analysis (Table 1).
Discussion: Our findings underscore clinicians’ abilities to down titrate tacrolimus levels without an associated increased risk of rejection. Higher tacrolimus levels were also not associated with an increased incidence of cancer, infections, nephrotoxicity, or hyperkalemia. This discussion may benefit from larger sample sizes for increased generalizability.

Figure: Table 1: Incidence of adverse effects by trough level
Note: The table for this abstract can be viewed in the ePoster Gallery section of the ACG 2024 ePoster Site or in The American Journal of Gastroenterology's abstract supplement issue, both of which will be available starting October 27, 2024.
Disclosures:
Abdulmalik Saleem indicated no relevant financial relationships.
Saleh Al-Juburi indicated no relevant financial relationships.
Ahmad Alomari indicated no relevant financial relationships.
Ammad Chaudhary indicated no relevant financial relationships.
Mohammed Abusuliman indicated no relevant financial relationships.
Muhammad Saad Faisal indicated no relevant financial relationships.
Haya Omeish indicated no relevant financial relationships.
Momin Samad indicated no relevant financial relationships.
Mohammed Nayeem indicated no relevant financial relationships.
Sheema Rehman indicated no relevant financial relationships.
Abu Fahad Abbasi indicated no relevant financial relationships.
Syed-Mohammed Jafri: Gilead, Takeda, Abbvie, Intercept, VectivBio – Advisor or Review Panel Member, Speakers Bureau.
Abdulmalik Saleem, MD1, Saleh Al-Juburi, BS2, Ahmad Alomari, MD3, Ammad Javaid. Chaudhary, MD1, Mohammed Abusuliman, MD1, Muhammad Saad Faisal, MD1, Haya Omeish, MD1, Momin Samad, MD4, Mohammed Nayeem, MD5, Sheema Rehman, DO3, Abu Fahad Abbasi, MD6, Syed-Mohammed Jafri, MD1. P1187 - Exploring the Limits: Minimizing Immunosuppression and Its Adverse Effects, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
