Sunday Poster Session
Category: Liver
P1266 - Severe Drug-Induced Liver Injury Due to Testolone, A Selective Androgen Receptor Modulator
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio
.jpg)
Sheel Patel, DO
University of Louisville
Louisville, KY
Presenting Author(s)
Sheel Patel, DO1, Aishwarya Thakurdesai, MD2, Dylan Flaherty, DO1, Navroop Nagra, MD1, Endashaw Omer, MD1, Kristine Krueger, MD1
1University of Louisville, Louisville, KY; 2University of Louisville School of Medicine, Louisville, KY
Introduction: Selective androgen receptor modulators (SARMs) are a novel class of androgen receptor ligands with tissue-selective anabolic effects and have therapeutic potential in conditions like muscular dystrophy, Alzheimer’s disease, and osteoporosis. Unlike traditional anabolic steroids, SARMs are thought to exert their action with reduced androgenic side effects, minimizing adverse effects. Testolone (RAD140) is a non-steroidal SARM that has recently gained popularity in the bodybuilding and fitness community for enhancing muscle growth and performance. While SARMs are thought to be safer than anabolic steroids, we present a case of drug-induced liver injury (DILI) from Testolone.
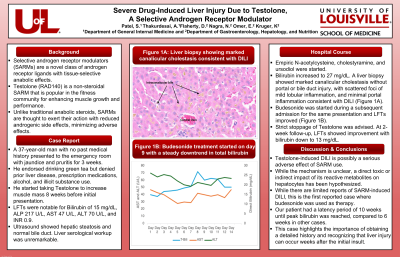
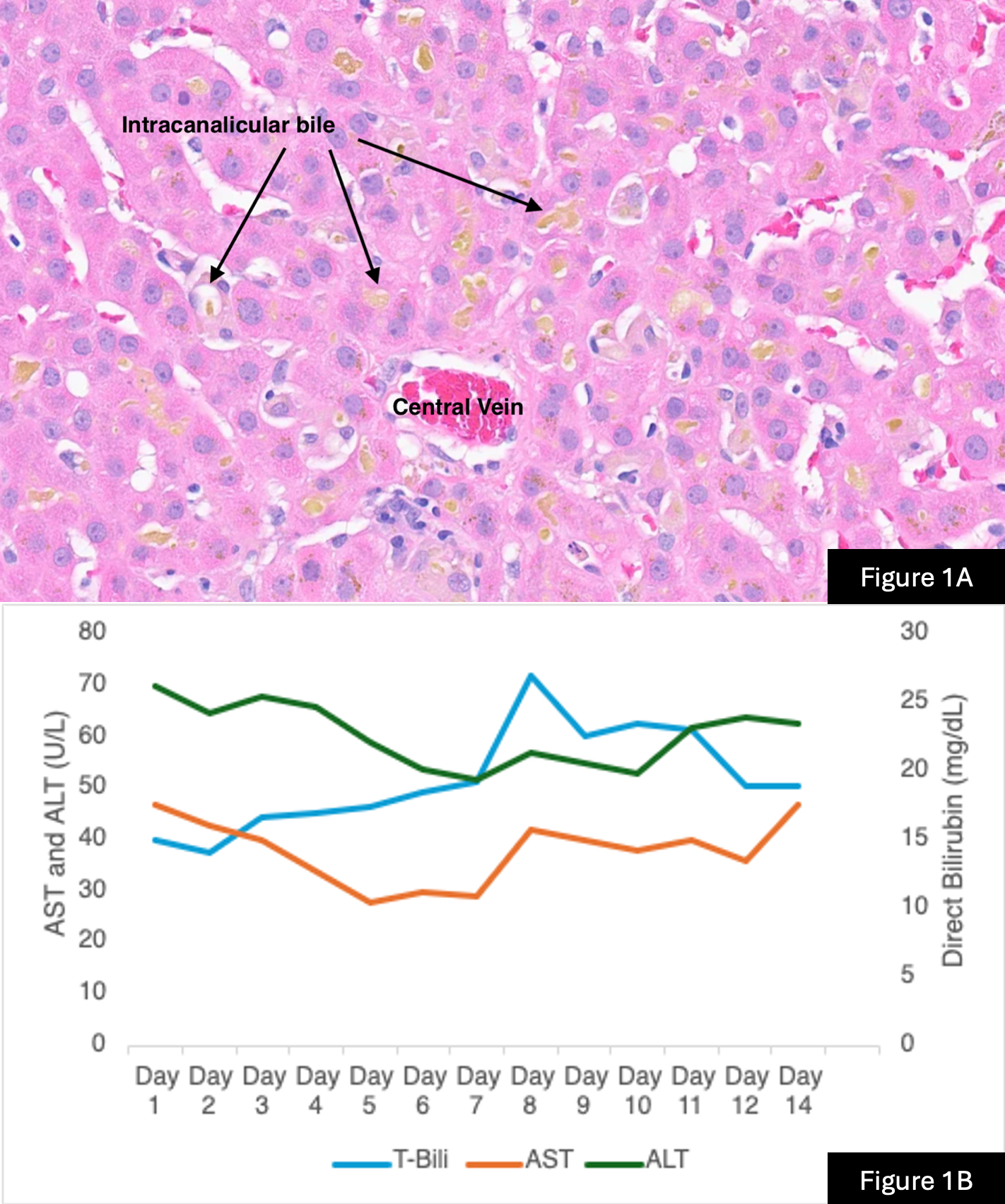
Case Description/Methods: A 37-year-old man with no past medical history presented to the emergency room (ER) with jaundice and pruritis for 3 weeks. He endorsed drinking green tea for years but denied prior liver disease, prescription medications, alcohol, and illicit substance use. He started taking Testolone to increase muscle mass 8 weeks before presenting to the ER. Liver function tests (LFTs) were notable for bilirubin of 15 mg/dL, ALP 217 U/L, AST 47 U/L, ALT 70 U/L, and INR 0.9. Ultrasound showed hepatic steatosis and normal bile duct. Liver serological workup was unremarkable for alternative etiologies of elevated liver enzymes. Empiric N-acetylcysteine, cholestyramine, and ursodiol were started. Bilirubin increased to 27 mg/dL. A liver biopsy showed marked canalicular cholestasis without portal or bile duct injury, with scattered foci of mild lobular inflammation, and minimal portal inflammation consistent with DILI (Figure 1A). Budesonide was started after admission for the same presentation and LFTs improved (Figure 1B). Strict stoppage of Testolone was advised. At 2-week follow-up, LFTs showed improvement with bilirubin down to 13 mg/dL.
Discussion: Testolone-induced DILI is possibly a serious adverse effect of SARM use. While the mechanism is unclear, a direct toxic or indirect impact of its reactive metabolites on hepatocytes has been hypothesized. RUCAM scale indicated that Testolone was the probable cause of DILI in our case. While there are limited reports of SARM-induced DILI, this is the first reported case where budesonide was used as therapy. Our patient had a latency period of 10 weeks until peak bilirubin was reached, compared to 6 weeks in other cases. This case highlights the importance of obtaining a detailed history and recognizing that liver injury can occur weeks after the initial insult.
Disclosures:
Sheel Patel, DO1, Aishwarya Thakurdesai, MD2, Dylan Flaherty, DO1, Navroop Nagra, MD1, Endashaw Omer, MD1, Kristine Krueger, MD1. P1266 - Severe Drug-Induced Liver Injury Due to Testolone, A Selective Androgen Receptor Modulator, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1University of Louisville, Louisville, KY; 2University of Louisville School of Medicine, Louisville, KY
Introduction: Selective androgen receptor modulators (SARMs) are a novel class of androgen receptor ligands with tissue-selective anabolic effects and have therapeutic potential in conditions like muscular dystrophy, Alzheimer’s disease, and osteoporosis. Unlike traditional anabolic steroids, SARMs are thought to exert their action with reduced androgenic side effects, minimizing adverse effects. Testolone (RAD140) is a non-steroidal SARM that has recently gained popularity in the bodybuilding and fitness community for enhancing muscle growth and performance. While SARMs are thought to be safer than anabolic steroids, we present a case of drug-induced liver injury (DILI) from Testolone.
Case Description/Methods: A 37-year-old man with no past medical history presented to the emergency room (ER) with jaundice and pruritis for 3 weeks. He endorsed drinking green tea for years but denied prior liver disease, prescription medications, alcohol, and illicit substance use. He started taking Testolone to increase muscle mass 8 weeks before presenting to the ER. Liver function tests (LFTs) were notable for bilirubin of 15 mg/dL, ALP 217 U/L, AST 47 U/L, ALT 70 U/L, and INR 0.9. Ultrasound showed hepatic steatosis and normal bile duct. Liver serological workup was unremarkable for alternative etiologies of elevated liver enzymes. Empiric N-acetylcysteine, cholestyramine, and ursodiol were started. Bilirubin increased to 27 mg/dL. A liver biopsy showed marked canalicular cholestasis without portal or bile duct injury, with scattered foci of mild lobular inflammation, and minimal portal inflammation consistent with DILI (Figure 1A). Budesonide was started after admission for the same presentation and LFTs improved (Figure 1B). Strict stoppage of Testolone was advised. At 2-week follow-up, LFTs showed improvement with bilirubin down to 13 mg/dL.
Discussion: Testolone-induced DILI is possibly a serious adverse effect of SARM use. While the mechanism is unclear, a direct toxic or indirect impact of its reactive metabolites on hepatocytes has been hypothesized. RUCAM scale indicated that Testolone was the probable cause of DILI in our case. While there are limited reports of SARM-induced DILI, this is the first reported case where budesonide was used as therapy. Our patient had a latency period of 10 weeks until peak bilirubin was reached, compared to 6 weeks in other cases. This case highlights the importance of obtaining a detailed history and recognizing that liver injury can occur weeks after the initial insult.

Figure: Figure 1A: Liver biopsy showing marked canalicular cholestasis consistent with DILI.
Figure 1B: Budesonide treatment started on day 9 demonstrating a steady downtrend in total bilirubin.
Figure 1B: Budesonide treatment started on day 9 demonstrating a steady downtrend in total bilirubin.
Disclosures:
Sheel Patel indicated no relevant financial relationships.
Aishwarya Thakurdesai indicated no relevant financial relationships.
Dylan Flaherty indicated no relevant financial relationships.
Navroop Nagra indicated no relevant financial relationships.
Endashaw Omer indicated no relevant financial relationships.
Kristine Krueger indicated no relevant financial relationships.
Sheel Patel, DO1, Aishwarya Thakurdesai, MD2, Dylan Flaherty, DO1, Navroop Nagra, MD1, Endashaw Omer, MD1, Kristine Krueger, MD1. P1266 - Severe Drug-Induced Liver Injury Due to Testolone, A Selective Androgen Receptor Modulator, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
