Sunday Poster Session
Category: Stomach
P1610 - Cost Reductions in Gastroesophageal Reflux Disease and Helicobacter pylori Treatments: Evaluating the Impact of MCCPDC on Medicare Expenditures
Sunday, October 27, 2024
3:30 PM - 7:00 PM ET
Location: Exhibit Hall E
Has Audio

Pratiksha Moliya, MD
Jamaica Hospital Medical Center
Edison, NJ
Presenting Author(s)
Pratiksha Moliya, MD1, Mohannad A. Bitar, MD2, Hima Varsha. Voruganti, MBBS, MD3, Hasan Al-Obaidi, MD4, Raj H. Patel, MD5, Charmy Parikh, MD6, Utsav Moliya, MBBS7, Oluwatayo J. Awolumate, MD8
1Jamaica Hospital Medical Center, Edison, NJ; 2Jamaica Hospital Medical Center, Queens, NY; 3North Alabama Medical Center, Florence, AL; 4Jamaica Hospital Medical Center, Briarwood, NY; 5St. Mary Medical Center, Bensalem, PA; 6Mercy Catholic Medical Center, Darby, PA; 7College of Medical Sciences, Bharatpur, Narayani, Nepal; 8Howard University Hospital, Washington, DC
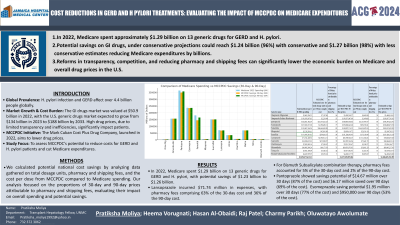
Introduction: H. Pylori infection and GERD are among the most common chronic GI conditions globally, affecting more than 4.4 billion people. The GI drugs market was valued at $50.9 billion in 2022, and the U.S. generic drugs market is expected to grow from $134 billion in 2023 to $188 billion by 2033. Despite this growth, high prices due to limited transparency and inefficiencies burden patients. Launched in 2022, the Mark Cuban Cost Plus Drug Company (MCCPDC) aims to reduce these costs. This study evaluates MCCPDC's potential to offer savings for GERD and H. pylori patients and to decrease Medicare expenditures.
Methods: We calculated potential national cost savings by analyzing data gathered on total dosage units, pharmacy and shipping fees, and the cost per dose from MCCPDC compared to Medicare spending. Our analysis focused on the proportions of 30-day and 90-day prices attributable to pharmacy and shipping fees, evaluating their impact on overall spending and potential savings.
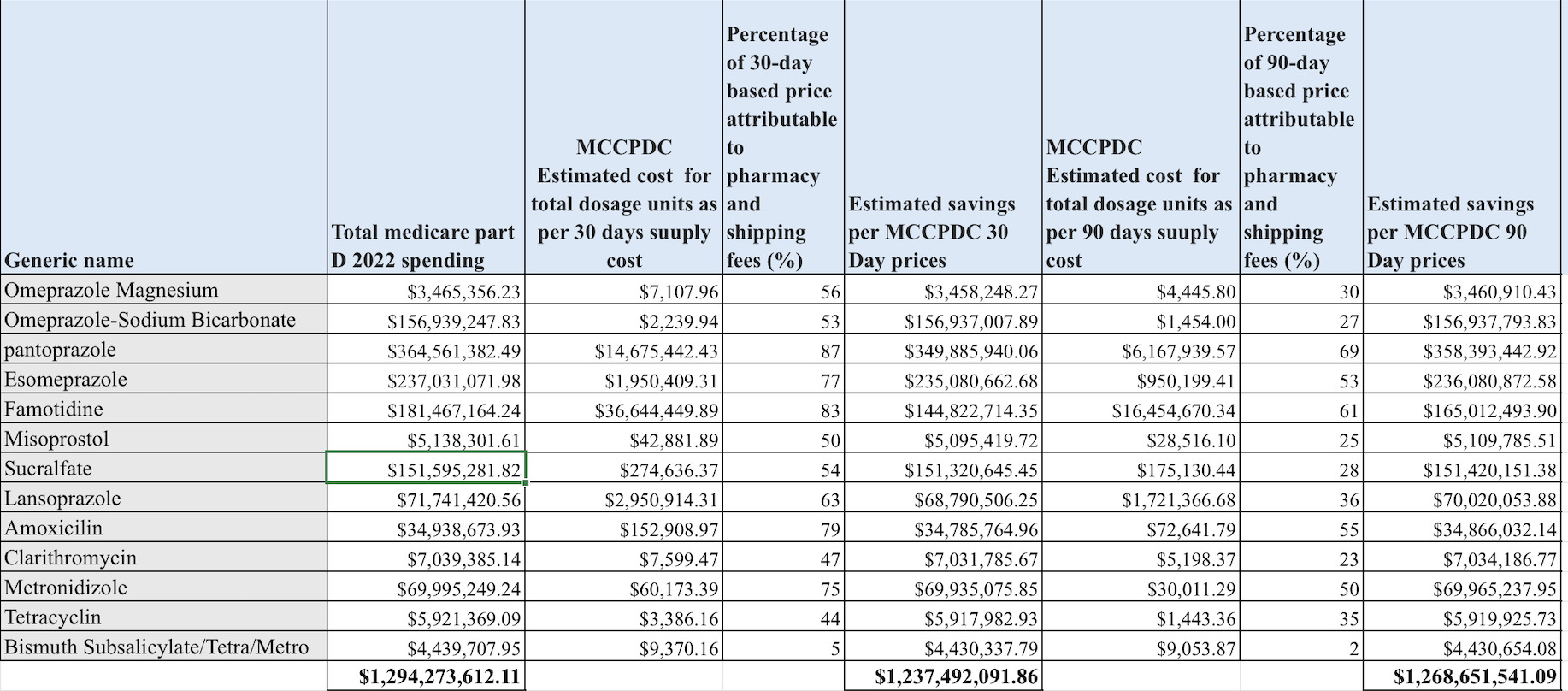
Results: In 2022, Medicare's expenditure on 13 generic drugs for GERD and H. pylori reached approximately $1.29 billion, with a substantial portion due to pharmacy and shipping costs. Our findings suggest savings of $1.23 million (96%) under conservative projections, and $1.26 million (98%) with less conservative estimates. Lansoprazole, a common GERD medication, incurred $71.74 million in expenses, with pharmacy fees comprising 63% of the 30-day price and 36% of the 90-day cost. Bismuth Subsalicylate combination therapy was particularly costly per dose, with pharmacy fees representing only 5% of the 30-day cost and 2% of the 90-day cost. Pantoprazole presented significant savings potential, with reductions of $14.67 million and $6.17 million over 30 and 90 days respectively, accounting for 87% and 69% of its total cost. Esomeprazole also showed savings opportunities, with potential pharmacy fee reductions of $1.95 million for 30 days and $950,000 for 90 days, representing 77% and 53% of the costs, respectively.
Discussion: he U.S. GI drug market can reduce costs by enhancing competition, cutting administrative expenses, and advocating for price transparency. MCCPDC offers cost-effective options for GERD and H. pylori treatments. This study underscores the need for policy reforms to tackle significant pharmacy and shipping fees that impact Medicare costs.
Disclosures:
Pratiksha Moliya, MD1, Mohannad A. Bitar, MD2, Hima Varsha. Voruganti, MBBS, MD3, Hasan Al-Obaidi, MD4, Raj H. Patel, MD5, Charmy Parikh, MD6, Utsav Moliya, MBBS7, Oluwatayo J. Awolumate, MD8. P1610 - Cost Reductions in Gastroesophageal Reflux Disease and <i>Helicobacter pylori</i> Treatments: Evaluating the Impact of MCCPDC on Medicare Expenditures, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
1Jamaica Hospital Medical Center, Edison, NJ; 2Jamaica Hospital Medical Center, Queens, NY; 3North Alabama Medical Center, Florence, AL; 4Jamaica Hospital Medical Center, Briarwood, NY; 5St. Mary Medical Center, Bensalem, PA; 6Mercy Catholic Medical Center, Darby, PA; 7College of Medical Sciences, Bharatpur, Narayani, Nepal; 8Howard University Hospital, Washington, DC
Introduction: H. Pylori infection and GERD are among the most common chronic GI conditions globally, affecting more than 4.4 billion people. The GI drugs market was valued at $50.9 billion in 2022, and the U.S. generic drugs market is expected to grow from $134 billion in 2023 to $188 billion by 2033. Despite this growth, high prices due to limited transparency and inefficiencies burden patients. Launched in 2022, the Mark Cuban Cost Plus Drug Company (MCCPDC) aims to reduce these costs. This study evaluates MCCPDC's potential to offer savings for GERD and H. pylori patients and to decrease Medicare expenditures.
Methods: We calculated potential national cost savings by analyzing data gathered on total dosage units, pharmacy and shipping fees, and the cost per dose from MCCPDC compared to Medicare spending. Our analysis focused on the proportions of 30-day and 90-day prices attributable to pharmacy and shipping fees, evaluating their impact on overall spending and potential savings.
Results: In 2022, Medicare's expenditure on 13 generic drugs for GERD and H. pylori reached approximately $1.29 billion, with a substantial portion due to pharmacy and shipping costs. Our findings suggest savings of $1.23 million (96%) under conservative projections, and $1.26 million (98%) with less conservative estimates. Lansoprazole, a common GERD medication, incurred $71.74 million in expenses, with pharmacy fees comprising 63% of the 30-day price and 36% of the 90-day cost. Bismuth Subsalicylate combination therapy was particularly costly per dose, with pharmacy fees representing only 5% of the 30-day cost and 2% of the 90-day cost. Pantoprazole presented significant savings potential, with reductions of $14.67 million and $6.17 million over 30 and 90 days respectively, accounting for 87% and 69% of its total cost. Esomeprazole also showed savings opportunities, with potential pharmacy fee reductions of $1.95 million for 30 days and $950,000 for 90 days, representing 77% and 53% of the costs, respectively.
Discussion: he U.S. GI drug market can reduce costs by enhancing competition, cutting administrative expenses, and advocating for price transparency. MCCPDC offers cost-effective options for GERD and H. pylori treatments. This study underscores the need for policy reforms to tackle significant pharmacy and shipping fees that impact Medicare costs.

Figure: Comparative Analysis of Medicare Part D Spending and MCCPDC Cost Estimates for Common GI Medications
Disclosures:
Pratiksha Moliya indicated no relevant financial relationships.
Mohannad Bitar indicated no relevant financial relationships.
Hima Voruganti indicated no relevant financial relationships.
Hasan Al-Obaidi indicated no relevant financial relationships.
Raj Patel indicated no relevant financial relationships.
Charmy Parikh indicated no relevant financial relationships.
Utsav Moliya indicated no relevant financial relationships.
Oluwatayo Awolumate indicated no relevant financial relationships.
Pratiksha Moliya, MD1, Mohannad A. Bitar, MD2, Hima Varsha. Voruganti, MBBS, MD3, Hasan Al-Obaidi, MD4, Raj H. Patel, MD5, Charmy Parikh, MD6, Utsav Moliya, MBBS7, Oluwatayo J. Awolumate, MD8. P1610 - Cost Reductions in Gastroesophageal Reflux Disease and <i>Helicobacter pylori</i> Treatments: Evaluating the Impact of MCCPDC on Medicare Expenditures, ACG 2024 Annual Scientific Meeting Abstracts. Philadelphia, PA: American College of Gastroenterology.
